Stiffness and surface topology of silicone implants competitively mediate inflammatory responses of macrophages and foreign body response
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
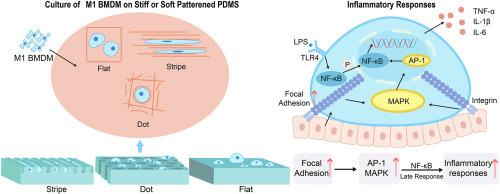
Adverse inflammatory responses, dominated by macrophages, that are induced by physical cues of silicone implants can heavily damage the life quality of patients via causing fibrosis and device failure. As stiffness and surface topology affect macrophages at the same time, the competition or partnership among physical cues against the regulation of macrophages is still ambiguous. Herein, a series of PDMS implants with different stiffness at ∼ MPa and surface topology at tens of micrometers were fabricated to investigate the relationship, the regulation rule, and the underlying mechanism of the two physical cues against the inflammatory responses of M1 macrophages. There is a competitive rule: surface topology could suppress the inflammatory responses of M1 macrophages in the soft group but did not have the same effect in the stiff group. Without surface topology, lower stiffness unexpectedly evoked stronger inflammatory responses of M1 macrophages. Implanting experiments also proved that the competitive state against mediating in vivo immune responses and the unexpected inflammatory responses. The reason is that stiffness could strongly up-regulate focal adhesion and activate the MAPK/NF-κB signaling axis to evoke inflammatory responses, which could shield the effect of surface topology. Therefore, for patient healthcare, it is crucial to prioritize stiffness while not surface topology at MPa levels to minimize adverse reactions.
硅胶植入体的硬度和表面拓扑结构竞争性地介导了巨噬细胞的炎症反应和异物反应
硅胶植入物的物理线索所诱发的以巨噬细胞为主的不良炎症反应会导致纤维化和装置失效,从而严重损害患者的生活质量。由于硬度和表面拓扑结构会同时影响巨噬细胞,物理线索对巨噬细胞调控的竞争或合作关系仍不明确。在此,我们制作了一系列刚度为 ∼ MPa、表面形貌为数十微米的 PDMS 植入物,以研究这两种物理线索对 M1 巨噬细胞炎症反应的影响关系、调控规则和内在机制。结果表明:表面拓扑结构在柔软组能抑制 M1 巨噬细胞的炎症反应,而在坚硬组则没有同样的效果。在没有表面拓扑结构的情况下,较低的硬度会意外地诱发更强的 M1 巨噬细胞炎症反应。植入实验还证明了竞争状态对调解体内免疫反应和意外的炎症反应的作用。原因是硬度会强烈上调病灶粘附并激活 MAPK/NF-κB 信号轴,从而唤起炎症反应,这可能会屏蔽表面拓扑结构的影响。因此,对于患者的医疗保健来说,至关重要的是要优先考虑硬度,而不是 MPa 级的表面拓扑结构,以尽量减少不良反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


