Rheological behavior of deep eutectic solvent promoted methane hydrate formation
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Natural gas hydrates are recognized as a crucial and sustainable energy resource. The formation, dissociation, and flow properties of CH4 hydrate from deep eutectic solvents (DESs) are investigated using a high-pressure rheometer. In this work, rheological experiments are performed using two DESs namely, tetrabutylammonium bromide + ethylene Glycol (TBAB + EG, DES1) and methyltriphenylphosphonium bromide + ethylene Glycol (MTPB + EG, DES2) at 1 wt% concentration, 274 K temperature and 8 MPa pressure. The hydrate formation starts around 1.7 h for DES1 and around 4 h for DES2. Pressure and viscosity of the slurry are analyzed while methane hydrate forms and dissociates. The pressure drop experienced during hydrate formation is roughly 1.4 MPa for DES1 and 1.7 MPa for DES2. Further, viscosity profiles are analyzed for both DESs with varying shear rate. The viscosity gradually decreases as the shear rate increases. At equilibrium conditions of pure CH4, a spike is observed during dissociation. The rheological data is further analyzed with Cross model to validate the shear-thinning behavior of methane hydrate.
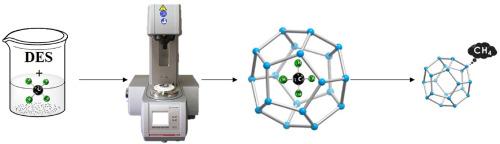
深共晶溶剂促进甲烷水合物形成的流变行为
天然气水合物被认为是一种重要的可持续能源资源。我们使用高压流变仪研究了深共晶溶剂(DES)中 CH4 水合物的形成、解离和流动特性。在这项工作中,使用两种 DES(即四丁基溴化铵 + 乙二醇(TBAB + EG,DES1)和甲基三苯基溴化膦 + 乙二醇(MTPB + EG,DES2))进行了流变实验,浓度为 1 wt%,温度为 274 K,压力为 8 MPa。DES1 在 1.7 小时左右开始形成水合物,DES2 在 4 小时左右开始形成水合物。在甲烷水合物形成和解离的过程中分析了浆液的压力和粘度。在水合物形成过程中,DES1 和 DES2 的压降分别约为 1.4 兆帕和 1.7 兆帕。此外,还分析了两种 DES 在不同剪切速率下的粘度曲线。随着剪切速率的增加,粘度逐渐降低。在纯 CH4 的平衡条件下,在解离过程中观察到一个尖峰。利用 Cross 模型对流变数据进行了进一步分析,以验证甲烷水合物的剪切稀化行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


