Structural modulation in colored polymer bands of methacrylic acid-based frontal polymerization
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The periodic colored polymer bands have been investigated in frontal polymerization (FP) reaction containing methacrylic acid (MAA), hydroquinone, Benzoyl peroxide (BP), and N, N Dimethyl aniline (DMA) system. The MAA acts as a monomer, which was diluted adequately with methanol (MeOH), ethanol (EtOH), and butanol (BuOH) separately to observe the patterning characteristics in different reaction schemes. The structural modulations that include the periodicity of colored polymer bands, morphological changes, and spacings between two adjacent layers were studied. The colored variations of polymer bands and their dependency on the concentration of BP, hydroquinone and alcoholic compositions have been studied. The highly consistent pink-white colored polymer bands, loosely-packed band structures, and perfectly ordered band structures resulted in MeOH, EtOH, and BuOH systems, respectively, as discussed. The evolution of tiny bubbles and convection-type instability has been observed in different conditions of the FP reactions that significantly affect the planar movement of the polymer fronts. The hot spots, which usually represent the high-temperature regions of a typical frontal surface, have also been demonstrated in all reacting systems, which may cause spin mode propagation of the fronts and change the surface geometry, as described. The materials characterization was carried out using UV–visible spectrophotometer, NMR, FTIR, and FESEM techniques, providing information about the polymer phases involved in band structures, composition, and surface properties. The analytical data and results obtained during the study further emphasized that two different colored polymers, namely 1, 4-dihydroxy anthraquinone methacrylic acid and poly-methacrylic acid dihydroxy, anthraquinone produced simultaneously and crystallized periodically, results in the development of a colored polymer band structures. The possible reactions and associated chemical mechanisms concerning the observations, the nature of the monomer, and solvent characteristics have been proposed, which show a close agreement with the analytical data and results obtained during the study.
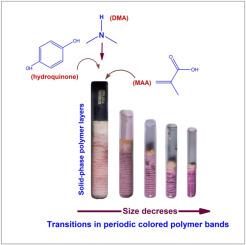
基于甲基丙烯酸的正面聚合的彩色聚合物带的结构调制
在含有甲基丙烯酸(MAA)、对苯二酚、过氧化苯甲酰(BP)和 N,N 二甲基苯胺(DMA)体系的正面聚合(FP)反应中,研究了周期性彩色聚合物带。MAA 作为单体,分别用甲醇 (MeOH)、乙醇 (EtOH) 和丁醇 (BuOH) 充分稀释,以观察不同反应方案中的图案特征。研究内容包括彩色聚合物带的周期性、形态变化和相邻两层之间的间距等结构变化。研究了聚合物色带的颜色变化及其与 BP、对苯二酚和酒精成分浓度的关系。分别讨论了在 MeOH、EtOH 和 BuOH 体系中产生的高度一致的粉白色聚合物带、松散堆积的带结构和完全有序的带结构。在 FP 反应的不同条件下,观察到了微小气泡和对流型不稳定性的演变,这极大地影响了聚合物前沿的平面运动。热点通常代表典型锋面的高温区域,在所有反应体系中也都得到了证实,这可能会导致锋面的自旋模式传播并改变表面几何形状,如前所述。材料表征采用了紫外-可见分光光度计、核磁共振、傅立叶变换红外光谱和 FESEM 技术,提供了有关带状结构、成分和表面特性所涉及的聚合物相的信息。研究中获得的分析数据和结果进一步强调了两种不同的有色聚合物,即 1,4-二羟基蒽醌甲基丙烯酸和聚甲基丙烯酸二羟基蒽醌同时生产并定期结晶,从而形成了有色聚合物带状结构。研究人员提出了与观察结果、单体性质和溶剂特性有关的可能反应和相关化学机制,这些反应和机制与分析数据和研究过程中获得的结果非常吻合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


