Synthesis, crystal structure, spectral analysis and NLO studies of five-coordinate Zn(II) complexes of hydrazochromandione
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Using a hydrazochromandione ligand, a straightforward chemical process is used to create zinc(II) complexes (1 and 2) with five coordination. Elemental microanalysis and a range of spectroscopic techniques (FT-IR, 1H NMR and electronic spectroscopy) were used to characterize the ligand and Zn(II) complexes. Single crystal X-ray diffraction was used to identify the crystal structures of HL, complex 1 and 2, and the results showed that ligand has monoclinic system with centrosymmetric space group P21/n. Through ONO donor atoms, Zn(II) metal is coordinated to the ligand in the same way as monoanionic. Two Zn(II) complexes with a deformed square pyramidal geometry surrounding them. Interestingly, relatively strong hydrogen bonds and p-p interactions that result in supramolecular architectures preserve the stabilization of the crystal lattices. Comparing two Zn(II) complexes to hydrazochromandione ligand, they exhibit good non-linear optical response.
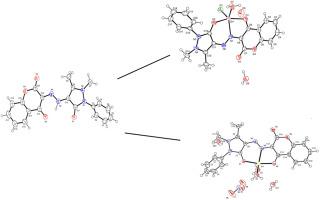
水合苯并二氢吡喃酮的五配位 Zn(II) 复合物的合成、晶体结构、光谱分析和 NLO 研究
该研究利用一种肼基苯并二氢吡喃二酮配体,通过简单的化学方法制备出具有五个配位的锌(II)配合物(1 和 2)。元素微量分析和一系列光谱技术(傅立叶变换红外光谱、1H NMR 和电子光谱)被用来表征配体和锌(II)配合物。利用单晶 X 射线衍射来确定 HL、复合物 1 和 2 的晶体结构,结果表明配体为单斜体系,中心对称空间群为 P21/n。金属锌(II)通过 ONO 供体原子与配体配位,配位方式与单阴离子配位方式相同。两个 Zn(II)配合物的周围存在变形的正方形金字塔几何结构。有趣的是,超分子结构中相对较强的氢键和 p-p 相互作用保持了晶格的稳定性。将两种锌(II)配合物与肼基色曼二酮配体进行比较,它们表现出良好的非线性光学响应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


