Redox-mediated synthesis of Cu2O/CuO/Mn3O4/C quaternary nanocomposites as an efficient electrode material for oxygen reduction reaction and supercapacitor application
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
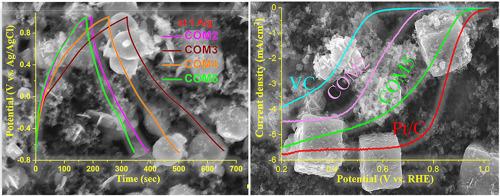
Earth-abundant transition metal oxides (TMOs) hold significant promise as electroactive materials in various electrochemical energy conversion and storage applications, offering economic and environmental advantages over their less abundant counterparts. In this work, a simple and cost-effective redox-mediated reaction strategy under hydrothermal conditions has been adopted to synthesize the quaternary nanocomposites Cu2O/CuO/Mn3O4/C with variable amounts of carbon. The synthesized nanocomposites have been characterized using various analysis techniques, including powder X-ray diffraction (PXRD), field emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR) and Brunauer–Emmett–Teller (BET). The synthesized COM5 nanocomposite (Cu2O/CuO/Mn3O4/C-50), when used as an electrocatalyst for the oxygen reduction reaction (ORR), demonstrates a good limiting current density (JL), half-wave potential (E1/2), and onset potential (Eonset) of −5.50 mA/cm2, 0.75 V, and 0.95 V, respectively, as per the polarization curve. The COM5 nanocomposite exhibits a four-electron transfer mechanism, calculated using the Koutecky-Levich (K-L) equation. In an O2-saturated 0.1M KOH solution, the COM5 nanocomposite has shown a superior relative current durability of 84.46% over 14,000 s compared to the commercially available 10 wt% Pt/C, indicating its potential application in the ORR. However, for supercapacitor applications, the COM3 nanocomposite (Cu2O/CuO/Mn3O4/C-20) as active electrode material in galvanic charge-discharge (GCD) study shows superior performance (201 F/g at 1 A/g) compared to the COM2 nanocomposite (117 F/g at 1 A/g) in neutral aqueous electrolyte, possibly due to the lower charge transfer resistance (Rct) of 24.04 Ω compared to COM2 (34.63 Ω). In two-electrode studies, the COM3 nanocomposite exhibits a good energy density of 24.19 Wh/kg at 1 A/g and a 4.7 kW/kg power density at 5 A/g. Additionally, the COM3 nanocomposite shows excellent long-term durability (74% capacitance retention) at the current density of 5 A/g for 8000 cycles. The electrochemical findings indicate that the COM3 nanocomposite stands out as a superior electrode material for supercapacitors, while the COM5 nanocomposite proves to be an effective electrocatalyst for the oxygen reduction reaction.
氧化还原介导合成 Cu2O/CuO/Mn3O4/C 季纳米复合材料,作为氧还原反应和超级电容器应用的高效电极材料
地球上丰富的过渡金属氧化物(TMOs)作为电活性材料在各种电化学能量转换和存储应用中大有可为,与储量较少的同类材料相比,具有经济和环境优势。本研究在水热条件下采用了一种简单而经济有效的氧化还原介导反应策略,合成了含有不同数量碳的四元纳米复合材料 Cu2O/CuO/Mn3O4/C。利用粉末 X 射线衍射 (PXRD)、场发射扫描电子显微镜 (FESEM)、高分辨率透射电子显微镜 (HRTEM)、X 射线光电子能谱 (XPS)、傅立叶变换红外光谱 (FTIR) 和布鲁瑙尔-艾美特-泰勒 (BET) 等多种分析技术对合成的纳米复合材料进行了表征。将合成的 COM5 纳米复合材料(Cu2O/CuO/Mn3O4/C-50)用作氧还原反应(ORR)的电催化剂时,根据极化曲线,其极限电流密度(JL)、半波电位(E1/2)和起始电位(Eonset)分别为 -5.50 mA/cm2、0.75 V 和 0.95 V。根据 Koutecky-Levich (K-L) 方程计算,COM5 纳米复合材料具有四电子转移机制。在氧气饱和的 0.1M KOH 溶液中,COM5 纳米复合材料与市售的 10 wt% Pt/C 相比,在 14,000 秒内的相对电流耐久性高达 84.46%,这表明它在 ORR 中具有潜在的应用价值。然而,在超级电容器应用方面,COM3 纳米复合材料(Cu2O/CuO/Mn3O4/C-20)作为活性电极材料,在电化学充放电(GCD)研究中,与 COM2 纳米复合材料(1 A/g,117 F/g)相比,COM3 纳米复合材料在中性水电解质中的性能(1 A/g,201 F/g)更优,这可能是由于电荷转移电阻(Rct)为 24.04 Ω,而 COM2 为 34.63 Ω。在双电极研究中,COM3 纳米复合材料表现出良好的能量密度,在 1 A/g 时为 24.19 Wh/kg,在 5 A/g 时为 4.7 kW/kg 功率密度。此外,在 5 A/g 的电流密度下,COM3 纳米复合材料在 8000 次循环中表现出卓越的长期耐久性(74% 的电容保持率)。电化学研究结果表明,COM3 纳米复合材料是超级电容器的理想电极材料,而 COM5 纳米复合材料则是氧还原反应的有效电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


