Integration of manganese-cobalt oxide nanoparticles into mesoporous Bi2WO6 n-n heterojunction for enhanced reductive removal of Hg(II) ions
IF 3.9
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Industrial contamination has harmed aquatic life by throwing huge quantities of toxic inorganic metals ions, especially Hg(II) ions into the ecosystem. Thus, it is critical demand to remove Hg species in industrial wastewater to match the safety standards. In this contribution, n-n heterojunction MnCo2O4/Bi2WO6 nanocomposites were fabricated using the sol–gel process utilizing Pluronic P-105 as a structure-directing agent. The photocatalytic Hg(II) reduction has been conducted over MnCo2O4/Bi2WO6 nanocomposites under illumination. The prepared MnCo2O4/Bi2WO6 photocatalysts exhibited better photocatalytic activity for reduction Hg(II) under visible illumination in comparison to bare Bi2WO6 NPs. Among the obtained photocatalysts, the optimum 6 %MnCo2O4/Bi2WO6 nanocomposite exhibited superior photocatalytic ability at about 100 % after 40 min. The rate constant of 6 %MnCo2O4/Bi2WO6 photocatalyst is 0.1177 min−1, which is almost 3.03 times greater than that of Bi2WO6 (0.0388 min−1). Moreover, the MnCo2O4/Bi2WO6 nanocomposite maintained its photocatalytic ability during five consecutive cycles. The mesostructure and S-scheme mechanism of MnCo2O4/Bi2WO6 nanocomposite promotes light absorption, high separation rate of carriers, and charge transport. The construction strategy of the obtained photocatalyst provides a feasible route for accelerating the practical abstraction of toxic Hg(II) ions.
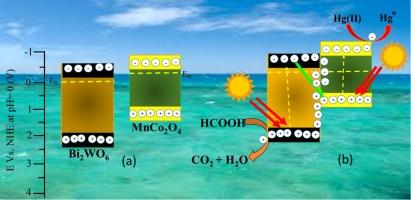
将锰-氧化钴纳米粒子集成到介孔 Bi2WO6 n-n 异质结中以增强对 Hg(II) 离子的还原去除能力
工业污染向生态系统中排放了大量有毒的无机金属离子,尤其是汞(II)离子,对水生生物造成了危害。因此,迫切需要去除工业废水中的汞,以达到安全标准。本研究采用溶胶-凝胶工艺,以 Pluronic P-105 为结构引导剂,制备了 n-n 异质结 MnCo2O4/Bi2WO6 纳米复合材料。在光照下,MnCo2O4/Bi2WO6 纳米复合材料进行了光催化还原汞(II)。与裸露的 Bi2WO6 纳米粒子相比,所制备的 MnCo2O4/Bi2WO6 光催化剂在可见光下具有更好的还原 Hg(II) 的光催化活性。在所获得的光催化剂中,最佳的 6 %MnCo2O4/Bi2WO6 纳米复合材料表现出更优越的光催化能力,40 分钟后的光催化率约为 100%。6%MnCo2O4/Bi2WO6 光催化剂的速率常数为 0.1177 min-1,几乎是 Bi2WO6(0.0388 min-1)的 3.03 倍。此外,MnCo2O4/Bi2WO6 纳米复合材料在连续五个周期内都能保持其光催化能力。MnCo2O4/Bi2WO6 纳米复合材料的介观结构和 S 型机制促进了光吸收、高载流子分离率和电荷传输。所获光催化剂的构建策略为加速有毒汞(II)离子的实际抽离提供了一条可行的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Science and Engineering: B
工程技术-材料科学:综合
CiteScore
5.60
自引率
2.80%
发文量
481
审稿时长
3.5 months
期刊介绍:
The journal provides an international medium for the publication of theoretical and experimental studies and reviews related to the electronic, electrochemical, ionic, magnetic, optical, and biosensing properties of solid state materials in bulk, thin film and particulate forms. Papers dealing with synthesis, processing, characterization, structure, physical properties and computational aspects of nano-crystalline, crystalline, amorphous and glassy forms of ceramics, semiconductors, layered insertion compounds, low-dimensional compounds and systems, fast-ion conductors, polymers and dielectrics are viewed as suitable for publication. Articles focused on nano-structured aspects of these advanced solid-state materials will also be considered suitable.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


