Oxalate-upregulated annexin A6 promotes the formation of calcium oxalate kidney stones by exacerbating calcium release-mediated oxidative stress injury in renal tubular epithelial cells and crystal-cell adhesion
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Kidney stones result from abnormal biomineralization, although the mechanism behind their formation remains unclear. Annexin A6 (AnxA6), a calcium-dependent lipid-binding protein, is associated with several mineralization-related diseases, but its role in kidney stones is unknown. This study aimed to explore the role and mechanism of AnxA6 in calcium oxalate (CaOx) kidney stones. An in vitro model in which renal tubular epithelial cells (RTECs) were treated with 1 mmol/L oxalate was established, and AnxA6 protein and mRNA expression were examined. Genetic engineering, drug intervention, and biochemical assays were used to investigate the role of AnxA6. The results revealed that AnxA6 was significantly overexpressed in the CaOx model. AnxA6 knockdown in RTECs reduced oxalate-induced oxidative stress, ROS accumulation, and mitochondrial damage, whereas AnxA6 overexpression exacerbated these effects. Blocking ryanodine receptor-mediated calcium release reversed AnxA6-induced oxidative damage. Additionally, AnxA6 increased oxalate adhesion to RTECs by binding to oxalate. In conclusion, AnxA6 contributes to CaOx kidney stone formation by promoting both oxidative stress via calcium release and crystal-cell adhesion by binding to oxalate. This study offers new insight into CaOx kidney stone formation.
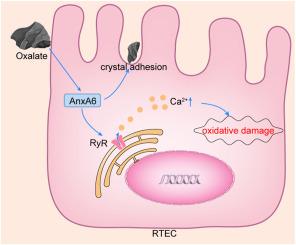
草酸盐上调的附件蛋白 A6 通过加剧钙释放介导的肾小管上皮细胞氧化应激损伤和晶体-细胞粘附,促进草酸钙肾结石的形成
肾结石是异常生物矿化的结果,但其形成机制仍不清楚。Annexin A6(AnxA6)是一种钙依赖性脂质结合蛋白,与多种矿化相关疾病有关,但在肾结石中的作用尚不清楚。本研究旨在探讨Annexin A6在草酸钙(CaOx)肾结石中的作用和机制。研究人员建立了一个体外模型,用 1 mmol/L 草酸盐处理肾小管上皮细胞(RTECs),并检测了 AnxA6 蛋白和 mRNA 的表达。研究人员采用基因工程、药物干预和生化试验等方法来研究 AnxA6 的作用。结果发现,AnxA6在CaOx模型中明显过表达。在 RTECs 中敲除 AnxA6 可减少草酸盐诱导的氧化应激、ROS 积累和线粒体损伤,而 AnxA6 的过表达则会加剧这些效应。阻断雷诺丁受体介导的钙释放可逆转 AnxA6 诱导的氧化损伤。此外,AnxA6 通过与草酸盐结合,增加了草酸盐对 RTEC 的粘附。总之,AnxA6 通过钙释放促进氧化应激,通过与草酸盐结合促进晶体细胞粘附,从而促进 CaOx 肾结石的形成。这项研究为了解 CaOx 肾结石的形成提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


