Interpretable machining learning assisted insights into bifunctional squaramide catalyzed ring-opening polymerization of lactide†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
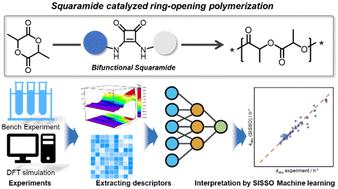
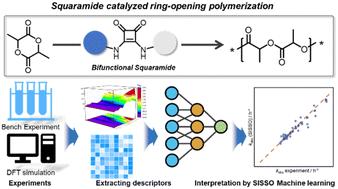
The structural understanding of catalysts is essential for achieving efficient and selective polymerization. In this study, we designed a series of bifunctional catalysts based on squaramide, carboxylates, and alkali cations for the ring-opening polymerization (ROP) of lactide. These catalysts exhibited controlled polymerization behavior with narrow dispersity (ĐM = 1.08–1.12). Kinetic evaluations revealed a linear relationship between the catalyst's chain length and activity for short CH2 chains (X = 1–4). However, as the CH2 segments lengthened, an “odd-even” effect on the kinetics was found, suggesting that the chain length alternately enhances or diminishes catalytic activity. The catalytic activity was significantly influenced by the counter cation (Li+, Na+, K+, and Cs+) of carboxylate, with larger radius cations showing higher rate constants (kobs Cs+ > kobs K+ > kobs Na+ > kobs Li+). Computational studies demonstrated that this correlation resulted from varying binding energies. Moreover, the kobs value of the catalyst can be tuned by adding different ratios of the crown ether. An interpretable machine learning method was introduced to link physical properties and activities, guiding the further design of effective catalysts for ROP.
双官能方酰胺催化的内酰胺开环聚合的可解释加工学习辅助见解
了解催化剂的结构对于实现高效和选择性聚合至关重要。在本研究中,我们设计了一系列基于方酰胺、羧酸盐和碱阳离子的双官能催化剂,用于内酰胺的开环聚合(ROP)。这些催化剂的聚合行为可控,分散度较窄(ĐM = 1.08-1.12)。动力学评估显示,催化剂的链长与短 CH2 链(X = 1-4)的活性之间存在线性关系。然而,随着 CH2 链段的加长,动力学上出现了 "奇偶 "效应,表明链长会交替增强或减弱催化活性。羧酸盐的反阳离子(Li+、Na+、K+ 和 Cs+)对催化活性有很大影响,半径较大的阳离子表现出更高的速率常数(kobs Cs+ > kobs K+ > kobs Na+ > kobs Li+)。计算研究表明,这种相关性源于不同的结合能。此外,还可以通过添加不同比例的冠醚来调整催化剂的 kobs 值。研究引入了一种可解释的机器学习方法,将物理性质和活性联系起来,从而指导进一步设计用于 ROP 的有效催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


