Characterization of PFOA isomers from PFAS precursors and their reductive defluorination
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
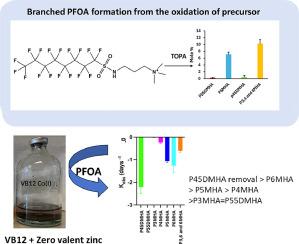
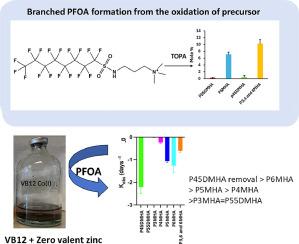
Perfluorooctanoic acid (PFOA) including linear and branched isomers is one of only three PFAS included in the Stockholm convention on Persistent Organic Pollutants. Unfortunately, PFOA branched isomers have received less attention than the linear due to analytical difficulties and perceived lower environmental concentrations. In this study, we revealed a environmentally relevant pathway for the formation of branched PFOA from PFAS precursors. AFFF samples showed a doubling of branched PFOA concentrations (138 mg/L) after TOP assay oxidation (307 mg/L). These findings indicate that branched PFOA may be more pervasive in the environment than previously thought. Additionally, we investigated the reductive degradability of PFOA using vitamin B12 (VB12) (a naturally occurring electron shuttle) in combination with either zero-valent zinc (ZVZ) or zero-valent iron (ZVI). Linear PFOA, as well as two branched isomers (3-methyl PFOA and 5,5-dimethyl PFOA), resisted reductive defluorination under the experimental conditions. However, all other branched isomers degraded within 10 days in the ZVZ-VB12 system. The experimental rate constants for specific PFOA isomers generally correlate with their calculated reduction potentials, except for 6-methyl PFOA. A potential defluorination pathway was proposed based on high-resolution mass spectrometry (LC-Orbitrap) and density functional theory (DFT) studies.
全氟辛烷磺酸前体中全氟辛烷磺酸异构体的特征及其还原脱氟反应
全氟辛酸(PFOA)包括直链异构体和支链异构体,是《关于持久性有机污染物的斯德哥尔摩公约》中仅有的三种全氟辛烷磺酸之一。遗憾的是,与线性异构体相比,支链异构体由于分析困难和环境浓度较低等原因,受到的关注较少。在这项研究中,我们揭示了支链全氟辛烷磺酸从全氟辛烷磺酸前体形成的环境相关途径。AFFF 样品在经过 TOP 分析氧化(307 毫克/升)后,支链全氟辛烷磺酸的浓度(138 毫克/升)增加了一倍。这些发现表明,支链全氟辛烷磺酸在环境中的存在可能比以前想象的更为普遍。此外,我们还利用维生素 B12(VB12)(一种天然存在的电子穿梭器)与零价锌(ZVZ)或零价铁(ZVI)结合,研究了 PFOA 的还原降解性。在实验条件下,线性全氟辛烷磺酸以及两种支链异构体(3-甲基全氟辛烷磺酸和 5,5-二甲基全氟辛烷磺酸)都能抵抗还原脱氟反应。然而,所有其他支链异构体在 ZVZ-VB12 系统中均在 10 天内降解。除 P6MHA 外,特定 PFOA 异构体的实验速率常数与其计算的还原电位基本相关。根据高分辨率质谱(LC-Orbitrap)和密度泛函理论(DFT)研究,提出了一种潜在的脱氟途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


