Exploring enzymatic degradation, reinforcement, recycling, and upcycling of poly(ester)-poly(urethane) with movable crosslinks
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Enzymes are highly efficient, chemoselective, and sustainable biocatalysts, standing out as eco-friendly tools to advance the circular plastics economy. Herein, we explored enzymatic reactions of poly(ε-caprolactone)-poly(urethane) (PCL-PUs) in organic solvent under different reaction conditions using Novozym 435 (immobilized lipase) as the enzyme. PCL-PUs with triacetylated γ-cyclodextrin (TAcγCD)-based movable crosslinks (PCL-γCD-PU) not only exhibited excellent mechanical properties due to effective energy dissipation, but also efficient enzymatic degradation that was optimized for increases in TAcγCD content. Under reaction time control, molecular weight and mechanical properties of PCL-γCD-PU were enhanced by a novel enzymatic reinforcement strategy. Without sorting, the degraded products are versatile resources that can be enzymatically closed-loop recycled by switching reaction concentration or enzymatically upcycled into value-added polymers by mixing with selective substrates. The facile polymer structure design combined with enzymatic reactions is expected to provide a broad approach for toughening various polymeric materials and advancing their development as sustainable resources.
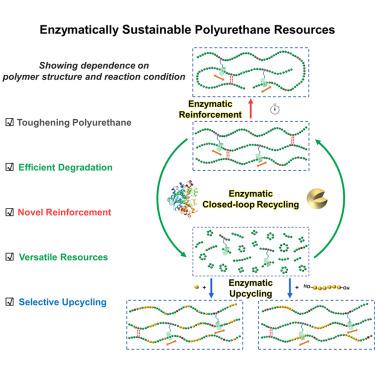

探索具有可移动交联的聚(酯)-聚(氨基甲酸乙酯)的酶降解、加固、回收和再循环
酶是一种高效、化学选择性和可持续的生物催化剂,是推动循环塑料经济的环保工具。在此,我们以 Novozym 435(固定化脂肪酶)为酶,探索了在不同反应条件下有机溶剂中聚(ε-己内酯)-聚(氨基甲酸乙酯)(PCL-PUs)的酶促反应。具有基于三乙酰化γ-环糊精(TAcγCD)的可移动交联的 PCL-PU(PCL-γCD-PU)不仅由于有效的能量耗散而表现出优异的机械性能,而且随着 TAcγCD 含量的增加,酶降解效果也得到了优化。在反应时间控制下,新型酶强化策略提高了 PCL-γCD-PU 的分子量和机械性能。降解产物无需分拣,是一种多功能资源,可通过转换反应浓度进行酶法闭环循环,或通过与选择性基质混合进行酶法升级循环,转化为高附加值聚合物。简便的聚合物结构设计与酶促反应相结合,有望为各种聚合物材料的增韧提供广泛的方法,并推动其作为可持续资源的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


