High‐Pressure‐Mediated Fragment Library Synthesis of 1,2‐Disubsituted Cyclobutane Derivatives
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Cyclobutanes have attracted significant interest in medicinal chemistry because of their unique structure and potential advantages in pharmacological properties. Nevertheless, 1,2‐disubstituted cyclobutanes remain underrepresented, both in the general chemical space and in fragment‐based drug discovery libraries. In this study, a two‐diversification‐point library of cyclobutanesulfonamides was synthesized through a hyperbaric [2+2] cycloaddition reaction between ethenesulfonyl fluoride and tert‐butyl vinyl ether as the key step. The sulfonyl fluoride was subsequently transformed into various sulfonamides, whereas the tert‐butyl ether was converted into carbamates and triazoles to synthesize a fragment library. Overall, this synthesis contributes to addressing the underrepresentation of 1,2‐disubstituted cyclobutane fragments, making a valuable addition to the field of fragment‐based drug discovery.
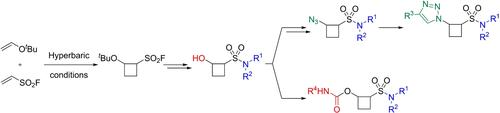

高压介导的 1,2-二取代环丁烷衍生物片段库合成
环丁烷因其独特的结构和潜在的药理特性优势,在药物化学领域引起了极大的兴趣。本研究以乙烯磺酰氟和叔丁基乙烯基醚之间的高压[2+2]环加成反应为关键步骤,合成了一个具有氨基甲酸酯或三唑的环丁烷磺酰胺类化合物的双扩散点文库。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


