Formation mechanism of Fe oxyhydroxides and behavior of metals during the oxidation of submarine sulfides at the Wocan-1 hydrothermal field, Carlsberg Ridge
IF 3.2
2区 地球科学
Q1 GEOLOGY
引用次数: 0
Abstract
Hydrothermal sulfides undergo complex oxidation process under seawater conditions, which has the potential to impact resource values and marine environments. However, the oxidation process is not fully explored, particularly regarding the transformation of minerals and the behavior of metals. In this paper, we conducted detailed mineralogical and geochemical analyses on a series of samples with varying degrees of oxidation, collected from the Wocan-1 hydrothermal field, Carlsberg Ridge, Northwest Indian Ocean. The principal purpose is to illuminate the formation and transformation of Fe (oxyhydr)oxides, and further to reveal the migration and redistribution of key metals throughout the oxidation process. We identified four types of Fe(−Si) (oxyhydr)oxides with two distinct formation mechanisms. Three Fe (oxyhydr)oxides are the direct oxidation products of pyrite and marcasite, while Fe-Si (oxyhydr)oxide precipitates from low-temperature hydrothermal fluids. Thin Fe (oxyhydr)oxide forms as the secondary product of euhedral pyrite at the early stage of oxidation. Pseudomorphic Fe (oxyhydr)oxide with low Fe contents, occurs as the replacement of euhedral marcasite. Filamentous Fe (oxyhydr)oxide, enriched in trace metals, is attributed to the uniform oxidation of subhedral pyrite-marcasite intergrowth. Moreover, major and trace elements occur multiple migrations and redistributions among primary sulfides, secondary products, and seawater. Notably, Fe (oxyhydr)oxides, through the sequestration mechanism, not only retain Cu and Zn released by the oxidation of primary sulfide minerals, but also scavenge Cu from seawater. However, the dissolution of Pb, As, Mo, and Co exceeds the amount retained during the oxidation, indicating that the potential release of toxic metals into the environment could pose a threat to the local ecosystem. These new insights can provide an initial foundation for the effect of submarine oxidation on economic values of sulfides and ecological environments of deep sea.
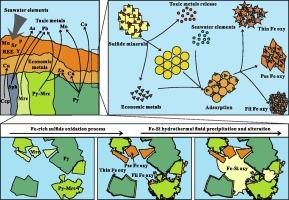
卡尔斯伯格海脊 Wocan-1 热液场海底硫化物氧化过程中铁氧氢氧化物的形成机制和金属的行为
热液硫化物在海水条件下会发生复杂的氧化过程,这有可能影响资源价值和海洋环境。然而,对氧化过程的探索还不够充分,特别是在矿物的转化和金属的行为方面。在本文中,我们对从西北印度洋卡尔斯伯格海脊 Wocan-1 热液场采集的一系列不同氧化程度的样本进行了详细的矿物学和地球化学分析。主要目的是阐明铁(氧氢)氧化物的形成和转化,并进一步揭示整个氧化过程中关键金属的迁移和再分布。我们发现了四种类型的铁(-Si)(氧水)氧化物,它们具有两种不同的形成机制。三种铁(氧水)氧化物是黄铁矿和云母石的直接氧化产物,而铁(-硅)(氧水)氧化物则是从低温热液中析出的。氧化铁(氧水)薄层是黄铁矿氧化初期的次生产物。含铁量较低的假象氧化铁(氧水),作为斜长石的置换物出现。富含微量金属的丝状铁(氧水)氧化物,是由于亚面体黄铁矿-水帘石间的均匀氧化作用而形成的。此外,主要元素和微量元素在原生硫化物、次生产物和海水中发生了多次迁移和再分布。值得注意的是,铁(氧氢)氧化物通过螯合机制,不仅保留了原生硫化物矿物氧化释放的铜和锌,还从海水中清除了铜。然而,铅、砷、钼和钴的溶解量超过了氧化过程中的截留量,这表明有毒金属可能会释放到环境中,对当地生态系统构成威胁。这些新见解可为研究海底氧化对硫化物经济价值和深海生态环境的影响提供初步依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Ore Geology Reviews
地学-地质学
CiteScore
6.50
自引率
27.30%
发文量
546
审稿时长
22.9 weeks
期刊介绍:
Ore Geology Reviews aims to familiarize all earth scientists with recent advances in a number of interconnected disciplines related to the study of, and search for, ore deposits. The reviews range from brief to longer contributions, but the journal preferentially publishes manuscripts that fill the niche between the commonly shorter journal articles and the comprehensive book coverages, and thus has a special appeal to many authors and readers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


