Highly dispersed Ru nanoparticles induced by the atmosphere for hydrogen production from ammonia decomposition
IF 5.5
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-10-28
DOI:10.1016/j.jtice.2024.105794
引用次数: 0
Abstract
Background
Ammonia (NH3) serves as a promising medium for hydrogen storage and transportation, addressing the challenges associated with these processes. However, the ammonia decomposition reaction urgently requires catalysts with high activity and stability.
Methods
Here, we present a synergistic strategy for the preparation of a highly dispersed Ru/Al2O3 catalyst using an atmosphere-induced method. Under an oxidizing atmosphere (Ru/Al2O3-O), the particle size of RuO2 exceeds 10 nm. Conversely, by oxygen vacancies anchoring in a reducing atmosphere, highly dispersed and structurally stable Ru catalysts with particle sizes < 2 nm can be prepared. HRTEM, XPS, TPR and XRD have been employed to elucidate the morphology and electronic structure of the Ru metal.
Significant Findings
This research investigated the impact of atmosphere-induced effects on the particle size and ammonia decomposition activity of Ru/γ-Al2O3 catalysts. A reducing atmosphere induced the formation of oxygen vacancies in the alumina support, leading to highly dispersed Ru/γ-Al2O3 catalysts. The interaction between Ru species and oxygen vacancies led to Ru particles of catalyst smaller than 2 nm and a stable structure. The removal of Cl ions from the Ru-based catalyst positively influenced the enhancement of ammonia decomposition activity. XPS and TPD results indicated that the introduction of the alkali metal potassium increased the electron density of Ru species, facilitating the ammonia decomposition reaction process. The K-Ru/γ-Al2O3-RW catalyst achieved a conversion rate of 97 % at 450 °C at a flow rate of 18,000 mL/gcat/h. Stability tests showed that the K-Ru/γ-Al2O3-RW catalyst did not deactivate after undergoing a 700-hour lifetime test. This work provides an effective method for synthesizing Ru-based catalysts to enhance ammonia decomposition for hydrogen production.
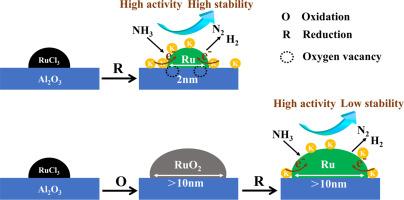
大气诱导的高度分散 Ru 纳米粒子用于氨分解制氢
背景氨气(NH3)是一种用于氢气储存和运输的前景广阔的介质,可解决与这些工艺相关的难题。然而,氨分解反应迫切需要具有高活性和稳定性的催化剂。在此,我们提出了一种利用气氛诱导法制备高分散 Ru/Al2O3 催化剂的协同策略。在氧化气氛(Ru/Al2O3-O)下,RuO2 的粒径超过 10 纳米。相反,通过在还原气氛中锚定氧空位,可以制备出粒径为 2 nm 的高度分散且结构稳定的 Ru 催化剂。本研究调查了大气诱导效应对 Ru/γ-Al2O3 催化剂粒度和氨分解活性的影响。还原气氛诱导氧化铝载体中氧空位的形成,导致 Ru/γ-Al2O3 催化剂高度分散。Ru 物种与氧空位之间的相互作用导致催化剂中的 Ru 颗粒小于 2 nm,且结构稳定。从 Ru 基催化剂中去除 Cl 离子对提高氨分解活性有积极影响。XPS 和 TPD 结果表明,碱金属钾的引入增加了 Ru 物种的电子密度,促进了氨分解反应过程。K-Ru/γ-Al2O3-RW 催化剂在 450 °C 温度和 18,000 mL/gcat/h 流速条件下的转化率达到了 97%。稳定性测试表明,K-Ru/γ-Al2O3-RW 催化剂在经历了 700 小时的寿命测试后没有失活。这项工作为合成 Ru 基催化剂提供了一种有效的方法,以提高氨分解制氢的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


