Enhanced oxygen evolution performance by single metal (tungsten, nickel and manganese) atom oxides anchored nanorods of CeO2-MnO2-rGO as electrocatalysts
IF 5.5
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-10-22
DOI:10.1016/j.jtice.2024.105800
引用次数: 0
Abstract
Background
In CeO2−MnO2-based electrocatalysts for oxygen evolution, addressing issues of stability and electron transfer delay is crucial for practical applications. The modification of electronic structures through single metal oxides (W, Ni, and Mn) can potentially enhance electron mobility and improve metal-support interactions, thus boosting electrocatalytic activity.
Method
To this end, CeO2−MnO2 nanorods intercalated with single metal atom oxides (SMAO) and supported by a reduced graphene oxide (rGO) layer (designated WNiMnCeMn-R-3) were synthesized using a sonication process.
Significant findings
This catalyst composition, particularly the WNiMnCeMn-R-3 variant with a CeO2 to MnO2 ratio of 15:45 %, exhibited significantly lower overpotential (280 mV) and Tafel slope (65.18 mV dec−1) at a current density of 10 mA cm−2 compared to other nanocomposites like CeO2−MnO2, CeO2−MnO2-rGO, WNiMnCeMn-R-1 (CeO2:MnO2 as 45:15 %) and WNiMnCeMn-R-2 (CeO2:MnO2 as 30:30 %). Exceptional electrochemical stability was demonstrated during a 24 h chronopotentiometry test over 2000 cyclic voltammetry cycles. The outstanding catalytic performance and stability of WNiMnCeMn-R-3 can be attributed to the synergistic effects of SMAO, CeO2, MnO2, and rGO layers, which collectively enhance the intrinsic catalytic activity and facilitate faster electron transport. This study aims to advance the development of electrochemical catalysts utilizing metal oxides, specifically SMAOs anchored onto rGO.
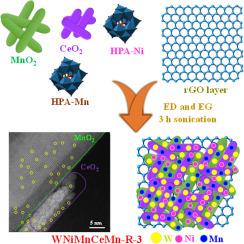
锚定 CeO2-MnO2-rGO 纳米棒的单一金属(钨、镍和锰)原子氧化物作为电催化剂可提高氧进化性能
背景在基于 CeO2-MnO2 的氧进化电催化剂中,解决稳定性和电子转移延迟问题对于实际应用至关重要。为此,我们采用超声工艺合成了插有单金属原子氧化物(SMAO)并由还原氧化石墨烯(rGO)层支撑的 CeO2-MnO2 纳米棒(命名为 WNiMnCeMn-R-3)。重要发现这种催化剂成分,特别是 CeO2 与 MnO2 之比为 15:45 % 的 WNiMnCeMn-R-3 变体,在催化活性条件下表现出明显较低的过电位(280 mV)和塔菲尔斜率(65.18 mV dec-1 )。在超过 2000 次循环伏安法周期的 24 小时计时电位计测试中,该催化剂表现出卓越的电化学稳定性。WNiMnCeMn-R-3 杰出的催化性能和稳定性可归因于 SMAO、CeO2、MnO2 和 rGO 层的协同作用,它们共同提高了催化活性并加快了电子传输速度。这项研究旨在推动利用金属氧化物,特别是锚定在 rGO 上的 SMAO 的电化学催化剂的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


