Facile synthesis of transition metal-selenides@CNTs for electrochemical oxygen evolution reactions
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
The fabrication of carbon-based efficient electrocatalysts for hydrogen production during electrochemical water splitting could be a great achievement to fulfill the future renewable energy demand. In this study, one-step hydrothermal process was used to develop composites of multi-metal selenides with carbon nanotubes (MnSe@CNTs, MnCuSe@CNTs, MnCoNiSe@CNTs) as electrocatalysts for OER during electrolytic water splitting. XRD and SEM with EDS analysis confirm the successful fabrication of the proposed catalyst and its crystalline morphology. Among the synthesized functionalized CNTs composites, bimetallic selenide composite gives a better electrocatalytic activity with reduced overpotential, lower Tafel slope and reduced charge transfer resistance in 1 M KOH. By using the catalyst(MnCuSe@CNTs) water oxidation starts at 1.4 V onset potential with a very low overpotential of 245 mV and the Tafel slope was only 107 mV dec−1. Metal-based selenides composites with CNTs introduce a lot of active sites, and subsequently nanoparticles of MnSe, MnCuSe, & MnCoNiSe, causes to boost the surface area and electrocatalysis toward oxygen evolution reactions.
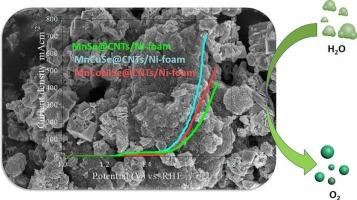
用于电化学氧进化反应的过渡金属硒化物@CNT的简便合成
制备碳基高效电催化剂用于电化学分水制氢是满足未来可再生能源需求的一项重大成就。本研究采用一步水热法制备了多金属硒化物与碳纳米管的复合材料(MnSe@CNTs、MnCuSe@CNTs、MnCoNiSe@CNTs),作为电解水分离过程中 OER 的电催化剂。XRD 和带有 EDS 的 SEM 分析证实了所提议的催化剂的成功制备及其结晶形态。在合成的功能化碳纳米管复合材料中,双金属硒化物复合材料具有更好的电催化活性,在 1 M KOH 中具有更低的过电位、更低的塔菲尔斜率和更低的电荷转移电阻。通过使用催化剂(MnCuSe@CNTs),水氧化在 1.4 V 的起始电位开始,过电位非常低,仅为 245 mV,塔菲尔斜率仅为 107 mV dec-1。金属基硒化物与 CNT 的复合引入了大量活性位点,随后 MnSe、MnCuSe、& MnCoNiSe 的纳米颗粒增加了比表面积,提高了对氧进化反应的电催化能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


