The unexpected and important role of alcohol stabilizer in thermal cis-trans isomerization of push-pull dyes in chloroform solution
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
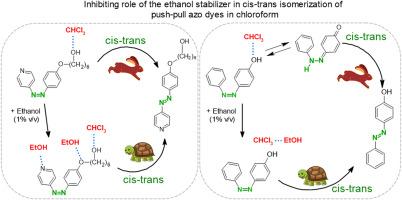
The study demonstrates the influence of ethanol as a stabilizer in chloroform on the thermal cis-trans isomerization kinetics of photochromic compounds based on azobenzene and azopyridine derivatives. The significance of the addition of ethanol as a stabilizer in chloroform-based solutions on the isomerization reaction of the hydroxy- and the 6-hydroxylalkoxy-substituted azo compounds was investigated. The results have revealed that the presence of a polar stabilizer in a solvent can significantly reduce the reaction rate constant of the dark cis-trans isomerization due to the formation of hydrogen bonds at both ends of the azo-chromophore, thereby inhibiting the isomerization. Furthermore, a stabilizer can facilitate the reversion of azo-hydrazone tautomers of azophenols to their azobenzene form, resulting in a decrease in the reaction rate constant. The research presented in this paper can be essential for scientists who study the kinetics of the trans-cis and the cis-trans isomerization reactions of azo-chromophores. The results show that the use of solvents with polar stabilizers should be rather avoided, as their usage without information about stabilizers can lead to different experimental data. The authors should ensure that detailed information about stabilizers used in unstable solvents is included in the experimental section of their works (a detail currently often omitted in the literature).
醇稳定剂在氯仿溶液中推挽染料热顺反异构化过程中的意外重要作用
该研究证明了乙醇作为氯仿中的稳定剂对基于偶氮苯和偶氮吡啶衍生物的光致变色化合物的热顺反异构动力学的影响。研究了在氯仿溶液中加入乙醇作为稳定剂对羟基和 6-羟基烷氧基取代偶氮化合物异构化反应的影响。研究结果表明,溶剂中极性稳定剂的存在可显著降低暗顺反异构反应的反应速率常数,这是由于偶氮色素团两端形成氢键,从而抑制了异构化反应。此外,稳定剂还能促进偶氮苯酚的偶氮腙同系物还原为偶氮苯形式,从而降低反应速率常数。对于研究偶氮色酚反式-顺式和顺式-反式异构化反应动力学的科学家来说,本文所介绍的研究非常重要。研究结果表明,应尽量避免使用带有极性稳定剂的溶剂,因为在不了解稳定剂信息的情况下使用这些溶剂会导致不同的实验数据。作者应确保在其作品的实验部分详细介绍不稳定溶剂中使用的稳定剂(目前文献中经常忽略这一细节)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


