Separation of yttrium from ion-adsorbed-rare-earth deposit leachates using N,N-di(2-ethylhexyl)-diglycolamic acid (HDEHDGA): Preliminary experimental and molecular dynamics simulation studies
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
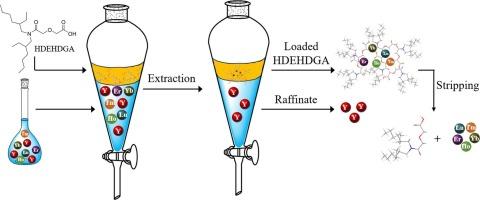
The separation of yttrium (Y) from heavy rare-earth elements (HREEs) is a major issue for ion-adsorbed rare-earth deposits. This study describes a new extraction system to develop efficient separation of Y from HREEs in a chloride medium using diglycolamic acid as the extractant. The extraction performance of N,N-di(2-ethylhexyl)-diglycolamic acid (HDEHDGA) for mixed REEs of an ion-adsorbed rare-earth deposit was investigated. The extraction order of the REEs in the HDEHDGA system followed a positive sequence, and the extraction behavior of Y resembled that of the middle REEs. Compared with naphthenic acid (NA) and sec-octylphenoxy acetic acid (CA12), HDEHDGA exhibited better separation performance for HREEs (Ho-Lu) and Y. The separation factors of Ho/Y, Er/Y, Tm/Y, Yb/Y, and Lu/Y in the Y-enriched solution were 4.41, 4.29, 3.77, 3.26, and 3.11, respectively. Combined slope analysis and electrospray ionization–high-resolution mass spectroscopy (ESI-HRMS) results identified cation exchange as the extraction mechanism of RE3+. Furthermore, the molecular dynamics simulation results provided new insights into the dynamic behaviors of Y3+ and Yb3+ extraction and revealed that the interaction of HDEHDGA with Yb3+ was stronger than that with Y3+. In addition, the loading capacity and recyclability of HDEHDGA were evaluated. This study highlights the potential of the HDEHDGA system for the separation of Y from HREEs.
使用 N,N-二(2-乙基己基)二甘醇氨基甲酸(HDEHDGA)从离子吸附的稀土矿床浸出液中分离钇:初步实验和分子动力学模拟研究
从重稀土元素(HREEs)中分离钇(Y)是离子吸附稀土矿床的一个主要问题。本研究介绍了一种新的萃取系统,该系统以二甘醇肟酸为萃取剂,在氯化物介质中开发出了从 HREEs 中高效分离 Y 的方法。研究了 N,N-二(2-乙基己基)-二甘醇胺酸(HDEHDGA)对离子吸附稀土矿床中混合 REEs 的萃取性能。在 HDEHDGA 体系中,REEs 的萃取顺序为正序,Y 的萃取行为与中间 REEs 相似。与环烷酸(NA)和仲辛基苯氧乙酸(CA12)相比,HDEHDGA对HREEs(Ho-Lu)和Y具有更好的分离性能。Y富集溶液中Ho/Y、Er/Y、Tm/Y、Yb/Y和Lu/Y的分离因子分别为4.41、4.29、3.77、3.26和3.11。结合斜率分析和电喷雾电离高分辨质谱(ESI-HRMS)结果,确定阳离子交换是 RE3+ 的萃取机制。此外,分子动力学模拟结果为 Y3+ 和 Yb3+ 萃取的动态行为提供了新的见解,并发现 HDEHDGA 与 Yb3+ 的相互作用强于与 Y3+ 的相互作用。此外,还评估了 HDEHDGA 的负载能力和可回收性。这项研究凸显了 HDEHDGA 系统从 HREEs 中分离 Y 的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


