Separation of hafnium from zircon leach solution using anion-exchange resin and production of high-purity zirconia for nuclear applications
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
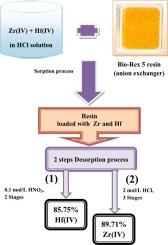
The ion exchange process using an anion exchange resin (Bio-Rex 5) was employed with batch and column techniques to isolate nuclear-grade zirconium/hafnium from a leach solution of zircon ore. Batch studies were conducted to optimize the conditions for sorption and desorption of Zr(IV) and Hf(IV). The highest separation factor of 10.3 was achieved under equilibrium conditions of 11.0 mol/L HCl, contact time of 30.0 min, solution volume-to-mass of resin ratio of 0.10, and 15 °C. The sorption process for both metals obeyed a pseudo-second-order model and the experimental sorption data was well-described by both Langmuir and Freundlich models. The maximum sorption capacities were determined to be 46.2 mg/g for Zr(IV) and 37.8 mg/g for Hf(IV). The Zr(IV) and Hf(IV) ions were effectively desorbed by 0.1 mol/L nitric and 2.0 mol/L hydrochloric acid solutions, respectively, with total yields of 89.7 % Zr(IV) and 85.8 % Hf(IV) via multistage desorption processes. In both batch and column techniques, the resin exhibited sorption selectivity for Zr and Hf over interfering elements in the hydrochloric acid leach solution of zircon sand. The loaded resin from real leach solution was subjected to desorption, zirconium sulfate precipitation, and calcination at 650 °C, resulting in a pure zirconia powder suitable for nuclear applications. This technique presents a promising effective method for the selective separation and recovery of high-purity zirconium and hafnium from their natural sources.
使用阴离子交换树脂从锆石浸出液中分离铪并生产核用高纯度氧化锆
利用阴离子交换树脂(Bio-Rex 5)的离子交换工艺,采用间歇式和柱式技术,从锆矿石浸出液中分离出核级锆/铪。进行了批处理研究,以优化锆(IV)和铪(IV)的吸附和解吸条件。在盐酸浓度为 11.0 摩尔/升、接触时间为 30.0 分钟、溶液体积与树脂质量比为 0.10 和温度为 15 ℃的平衡条件下,分离系数最高,达到 10.3。两种金属的吸附过程都遵循伪二阶模型,实验吸附数据都能很好地用 Langmuir 和 Freundlich 模型来描述。经测定,Zr(IV) 的最大吸附容量为 46.2 mg/g,Hf(IV) 的最大吸附容量为 37.8 mg/g。通过多级解吸过程,Zr(IV) 和 Hf(IV) 离子分别被 0.1 mol/L 硝酸和 2.0 mol/L 盐酸溶液有效解吸,Zr(IV) 和 Hf(IV) 的总产率分别为 89.7% 和 85.8%。在间歇式和柱式技术中,树脂对锆英砂盐酸浸出液中的 Zr 和 Hf 的吸附选择性均优于干扰元素。真正浸出液中的负载树脂经过解吸、硫酸锆沉淀和 650 °C 煅烧后,得到了适合核应用的纯氧化锆粉末。这项技术为从天然来源中选择性分离和回收高纯度锆和铪提供了一种很有前景的有效方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


