Augmented glycerosomes as a promising approach against fungal ear infection: Optimization and microbiological, ex vivo and in vivo assessments
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
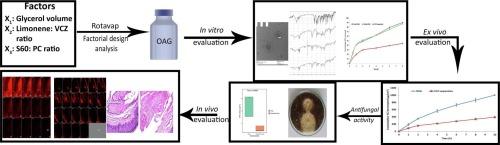
In the current study, voriconazole (VCZ) augmented glycerosomes were optimized for topical otomycosis management according to a 23 factorial design, employing a thin film hydration method. By optimizing Glycerol volume, limonene: VCZ ratio and Span® 60: soybean phosphatidyl choline (PC) ratio, glycerosomes with maximum percentage entrapment efficiency (%EE) and zeta potential (ZP) and minimum vesicle size (VS) and polydispersity index (PDI) were to be obtained. An optimal augmented glycerosomal formula (OAG) that contained 10 mg VCZ, 150 mg PC, and 3 mL glycerol, comprising 2.5: and 0.92:1 ratios of the latter two independent variables, was proposed via numerical optimization. OAG exhibited high %EE and ZP values and acceptable low values for VS and PDI (84.3 ± 2.0 %, −38.8 ± 1.8 mV, 191.0 ± 1.1 nm, and 0.192 ± 0.01, respectively). Extensive in vitro testing of OAG revealed the entrapment of VCZ within OAG, biphasic in vitro release profile, stability for up to 3 months at 2–8 °C and spherical morphology of OAG with VS like that obtained via zetasizer. OAG demonstrated higher permeated amounts of VCZ and flux values than VCZ suspension, leading to an enhancement ratio of 2.56 in the ex vivo permeation study. The deeper penetration ability of OAG demonstrated by Confocal Laser Scanning Microscopy and its superior in vitro antifungal activity confirmed the validity of the ex vivo study. Also, the histopathological study confirmed the safety of OAG for topical use, suggesting that VCZ OAG was a promising topical antimycotic formula.
增效甘油三酯体是一种很有前景的抗真菌耳部感染的方法:优化及微生物、体内外评估
在本研究中,采用薄膜水合法,根据 23 个因子设计,优化了用于局部治疗耳霉菌病的伏立康唑(VCZ)增效甘油三酯体。通过优化甘油体积、柠檬烯60:大豆磷脂酰胆碱(PC)的比例,从而获得夹带效率(%EE)和ZP(ZETA电位)最大、囊泡尺寸(VS)和多分散指数(PDI)最小的甘油囊体。通过数值优化,提出了一种最佳增强甘油囊配方(OAG),其中包含 10 毫克 VCZ、150 毫克 PC 和 3 毫升甘油,后两个自变量的比例分别为 2.5:1 和 0.92:1。OAG 显示出较高的 %EE 和 ZP 值,以及可接受的较低 VS 和 PDI 值(分别为 84.3 ± 2.0 %、-38.8 ± 1.8 mV、191.0 ± 1.1 nm 和 0.192 ± 0.01)。对 OAG 进行的大量体外测试表明,VCZ 在 OAG 中被包裹,体外释放曲线呈双相,在 2 2-8 °C 下可稳定 3 个月,OAG 呈球形,其 VS 与通过zetasizer 获得的相似。与 VCZ 悬浮液相比,OAG 表现出更高的 VCZ 渗透量和通量值,因此在体内外渗透研究中的增强比为 2.56。共焦激光扫描显微镜显示了 OAG 的深层渗透能力,其优异的体外抗真菌活性也证实了体内外研究的有效性。此外,组织病理学研究也证实了 OAG 局部使用的安全性,表明 VCZ OAG 是一种很有前途的局部抗真菌配方。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


