Study on the preparation of stabilizer-free silymarin nanocrystals and its oral absorption mechanisms
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Many researchers have studied the oral absorption mechanisms yet, however, considering stabilizers often participate in the absorption process of nanocrystals, these known mechanisms may be incorrect. Hence in this study, we aimed to explore the correct absorption mechanism of nanocrystals by performing related studies on stabilizer-free nanocrystals. We firstly prepared stabilizer-free silymarin nanocrystals by high-pressure homogenization, and then performed absorption-related studies, such as solubility, dissolution rate, pharmacokinetic study, cellular uptake and intracellular transport. Results showed the stabilizer-free silymarin nanocrystals had an average particle size of (450.2 ± 4.46) nm, with PDI of 0.280 ± 0.021 and Zeta potential of −26.9 ± 2.4 mV. The conversion of silymarin crude drug to stabilizer-free silymarin nanocrystals increased the compound's solubility by 1.41 times, with a dissolution rate of 92.2 % in water within 30 min compared to 38.5 % for crude drugs. Pharmacokinetic studies showed the oral bioavailability of stabilizer-free silymarin nanocrystals was found to be 1.48 times greater than that of the crude drugs. The cell experimentation results demonstrated that the stabilizer-silymarin nanocrystals can improve uptake but have poor transmembrane transport properties. Most researchers believe that nanocrystals can enhance transmembrane transport of drugs via an endocytosis-mediated pathway. In fact, nanocrystals are indeed endocytosed more by the cells, but this transport pathway is poor because the cells lack the intracellular transport pathway to transport nanocrystals from the AP side to the BP side. Therefore, we believe that the intracellular transport of nanocrystals can be enhanced by modifications and other carriers if needed to improve nanocrystals' ability to promote oral absorption.
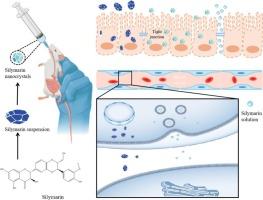
无稳定剂水飞蓟素纳米晶体的制备及其口服吸收机制研究
然而,考虑到稳定剂通常会参与纳米晶体的吸收过程,这些已知机制可能并不正确。因此,本研究旨在通过对不含稳定剂的纳米晶体进行相关研究,探索纳米晶体的正确吸收机制。我们首先通过高压均质法制备了不含稳定剂的水飞蓟素纳米晶体,然后进行了吸收相关研究,如溶解度、溶出率、药代动力学研究、细胞摄取和细胞内转运等。结果表明,不含稳定剂的水飞蓟素纳米晶体的平均粒径为(450.2 ± 4.46)nm,PDI为0.280 ± 0.021,Zeta电位为-26.9 ± 2.4 mV。将水飞蓟素原药转化为不含稳定剂的水飞蓟素纳米晶体后,该化合物的溶解度提高了 1.41 倍,30 分钟内在水中的溶解度为 92.2%,而原药的溶解度仅为 38.5%。药代动力学研究表明,不含稳定剂的水飞蓟素纳米晶体的口服生物利用度是原药的 1.48 倍。细胞实验结果表明,含稳定剂的水飞蓟素纳米晶体能提高吸收率,但跨膜转运性能较差。大多数研究人员认为,纳米晶体可通过内吞途径提高药物的跨膜转运。事实上,纳米晶体确实更多地被细胞内吞,但这种转运途径很差,因为细胞缺乏将纳米晶体从 AP 侧转运到 BP 侧的胞内转运途径。因此,我们认为,如果需要,可以通过改性和其他载体来增强纳米晶体的胞内转运,从而提高纳米晶体促进口服吸收的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
文献相关原料
公司名称
产品信息
索莱宝
PBS buffer
阿拉丁
Verapamil
阿拉丁
Chlorpromazine
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


