Bimetallic FeCo metal–organic framework based cascade reaction system: Enhanced peroxidase activity for antibacterial performance
IF 5.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
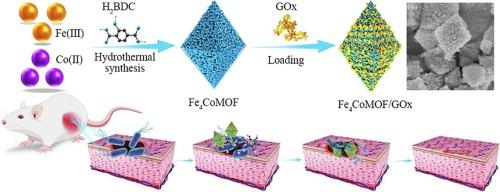
Metal-organic frameworks (MOFs), as a peroxidase (POD), can catalyze the conversion of H2O2 to reactive oxygen species (ROS) for antibacterial application. To achieve strong antibacterial activity, it is necessary to improve the enzyme-like activity of MOFs. In this study, FexCoMOF was synthesized by incorporating Co(II) to improve the electron transfer of Fe(III)/Fe(II), which enhanced its redox capacity as a nanozyme and further promoted the generation of hydroxyl radical (⋅OH), exhibiting higher POD activity. Doping with different amounts of Co(II) altered the particle size, specific surface area, and surface defects of FexCoMOF, thus exhibiting differential enzyme-like activities. Additionally, a glucose-responsive enzyme cascade reaction system based on Fe4CoMOF/glucose oxidase (GOx) was established. In the presence of glucose, the in situ-generated substrate H2O2 was in contact with the catalytic site and the generated gluconic acid enabled Fe4CoMOF to maintain maximum enzyme activity under physiological pH conditions, avoiding damage caused by the use of exogenous high concentrations of H2O2. In the in vitro antibacterial experiment, the minimum inhibitory concentration (MIC) of Fe4CoMOF/GOx was 10 μg mL−1 for Escherichia coli (E. coli) and 5 μg mL−1 for Staphylococcus aureus (S. aureus) (∼108 CFU mL−1). The increase in enzyme activity resulted in a considerable reduction in the dose of the antibacterial agent, which was conducive to improving bio-safety. The in vivo experiment in mice demonstrated that the glucose-responsive Fe4CoMOF-based cascade reaction system had excellent antibacterial properties and remarkably promoted wound healing. The antibacterial agent based on bimetallic MOF developed in this work provides a new idea for cascade catalytic antibacterial therapy and will have remarkable application prospects in biomaterials and nanomedicine.
基于级联反应系统的双金属 FeCo 金属有机框架:增强过氧化物酶活性以提高抗菌性能
金属有机框架(MOFs)作为一种过氧化物酶(POD),可以催化 H2O2 向活性氧(ROS)的转化,从而达到抗菌的目的。要实现较强的抗菌活性,就必须提高 MOFs 的类酶活性。本研究通过加入 Co(II) 来改善 Fe(III)/Fe(II) 的电子传递,合成了 FexCoMOF,从而增强了其作为纳米酶的氧化还原能力,并进一步促进了羟基自由基(⋅OH)的生成,表现出更高的 POD 活性。掺入不同量的 Co(II)改变了 FexCoMOF 的粒径、比表面积和表面缺陷,从而表现出不同的酶样活性。此外,还建立了基于 Fe4CoMOF/葡萄糖氧化酶(GOx)的葡萄糖响应酶级联反应系统。在葡萄糖存在的情况下,原位生成的底物 H2O2 与催化位点接触,生成的葡萄糖酸使 Fe4CoMOF 在生理 pH 条件下保持最大的酶活性,避免了使用外源高浓度 H2O2 造成的损害。在体外抗菌实验中,Fe4CoMOF/GOx 对大肠杆菌(E. coli)的最小抑菌浓度(MIC)为 10 μg mL-1,对金黄色葡萄球菌(S. aureus)的最小抑菌浓度(MIC)为 5 μg mL-1(108 CFU mL-1)。酶活性的提高大大减少了抗菌剂的剂量,有利于提高生物安全性。小鼠体内实验表明,基于葡萄糖响应的 Fe4CoMOF 级联反应体系具有优异的抗菌性能,并能显著促进伤口愈合。该研究开发的基于双金属MOF的抗菌剂为级联催化抗菌治疗提供了新思路,在生物材料和纳米医学领域具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Arabian Journal of Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
10.80
自引率
3.30%
发文量
763
审稿时长
63 days
期刊介绍:
The Arabian Journal of Chemistry is an English language, peer-reviewed scholarly publication in the area of chemistry. The Arabian Journal of Chemistry publishes original papers, reviews and short reports on, but not limited to: inorganic, physical, organic, analytical and biochemistry.
The Arabian Journal of Chemistry is issued by the Arab Union of Chemists and is published by King Saud University together with the Saudi Chemical Society in collaboration with Elsevier and is edited by an international group of eminent researchers.
文献相关原料
公司名称
产品信息
阿拉丁
terephthalic acid
阿拉丁
glucose (Glu)
阿拉丁
H2O2
阿拉丁
3,3′,5,5′-tetramethylbenzidine
阿拉丁
cobalt nitrate hexahydrate (Co(NO3)2·6H2O)
阿拉丁
Iron chloride hexahydrate (FeCl3·6H2O)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


