NiCo2O4 loaded on jute fiber porous carbon as an anode material for lithium-ion batteries
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
With the continuous development of science and technology, there is an increasing demand for batteries, and lithium-ion batteries (LIBs) are favored due to their compact size, strong energy storage capacity, long cycle life, lack of memory effect, and environmental friendliness, etc. The high theoretical specific capacity of NiCo2O4 makes it a promising material for an anode. However, it has many drawbacks, including a slow ion transfer rate and an easy way for volume to expand during charging and discharging. To address these limitations, we have developed a composite material. Jute fiber porous carbon was successfully prepared by high-temperature activation using jute fiber as the carbon matrix and potassium carbonate as the activator, and NiCo2O4 was loaded onto the jute fiber porous carbon and named NiCo2O4@JFPC. The jute fiber porous carbon serves as a carbon matrix that effectively improves the electrical conductivity of the composites while also slowing down the volume expansion caused by the frequent embedding/de-embedding of lithium ions, and at the same time, providing more effective paths for the transport of lithium ions. The NiCo2O4@JFPC composites were synthesized by solvothermal method. The effects of varying calcination temperatures (250, 350, and 450 °C) on the properties of the composites were investigated. The discharge capacity of NiCo2O4@JFPC-350 reached 1217.9 mAh g−1 after 100 cycles at 0.2C at a calcination temperature of 350 °C.
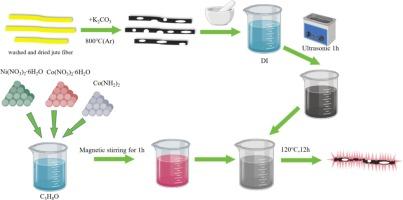
负载在黄麻纤维多孔碳上的镍钴氧化物作为锂离子电池的负极材料
随着科学技术的不断发展,人们对电池的需求越来越大,而锂离子电池(LIB)因其体积小、储能能力强、循环寿命长、无记忆效应、环保等优点而备受青睐。镍钴氧化物具有很高的理论比容量,因此是一种很有前途的负极材料。然而,它也有许多缺点,包括离子传输速率慢,充放电过程中体积容易膨胀。为了解决这些局限性,我们开发了一种复合材料。以黄麻纤维为碳基体,碳酸钾为活化剂,通过高温活化成功制备了黄麻纤维多孔碳,并将镍钴氧化物负载到黄麻纤维多孔碳上,命名为镍钴氧化物@JFPC。黄麻纤维多孔碳作为碳基体,在有效提高复合材料导电性能的同时,也减缓了锂离子频繁嵌入/脱嵌引起的体积膨胀,同时为锂离子的传输提供了更有效的路径。镍钴氧化物@JFPC复合材料是通过溶热法合成的。研究了不同煅烧温度(250、350 和 450 ℃)对复合材料性能的影响。在煅烧温度为 350 °C 时,镍钴氧化物@JFPC-350 在 0.2C 下循环 100 次后,放电容量达到 1217.9 mAh g-1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


