Oxazaborolidine catalyzed asymmetric 1,4-addition of diarylphosphine oxides to α,β-unsaturated N-acylindoles and N-acylpyrroles
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The enantioselective 1,4-addition of diarylphosphine oxides to α,β-unsaturated N-acylindoles and N-acylpyrroles catalyzed by a commercial chiral oxazaborolidine is reported. This methodology tolerates a variety of substrates and diarylphosphine oxides, leading to high yields and enantioselectivities. Importantly, the resulting chiral products exhibit excellent transformation abilities to form corresponding acids, amides, and esters with high potential applications in the synthesis of chiral phosphine ligands.
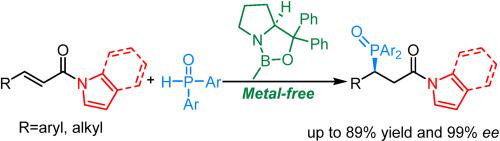
噁唑硼烷催化二芳基膦氧化物与 α、β-不饱和 N-酰基吲哚和 N-酰基吡咯的不对称 1,4-加成反应
报告了在一种商用手性噁唑硼烷催化下,二芳基膦氧化物与 α、β-不饱和 N-酰基吲哚和 N-酰基吡咯的 1,4-对映体选择性加成。该方法可耐受多种底物和二元膦氧化物,从而获得高产率和高对映选择性。重要的是,所得到的手性产物具有极佳的转化能力,可以形成相应的酸、酰胺和酯,在手性膦配体的合成中具有极高的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


