Synthesis of novel isostere analogues of naphthyridines using CuI catalyst: DFT computations (FMO, MEP), molecular docking and ADME analysis
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An approach towards the synthesis of novel isosteres of benzonaphthyridines and benzonaphthonaphthyridines from the condensation reaction between 4-chloro-2-methylquinolines/4-chloro-2-methylbenzo[h]quinoline and appropriate o-amino aromatic and heteroaromatic carboxylic acids by using solvent (ethanol)/solvent free (neat) condition to yield the intermediate followed by the cyclization with PPA. The intermediate yield has been slightly increased in neat (solvent-free) conditions compared to solvent conditions. Further, the target isosteres of benzonaphthyridines and benzonaphthonaphthyridines were achieved in the one-pot synthesis using a CuI catalyst with a higher yield than the stepwise method. Quantum chemical calculations of synthesized compounds are performed by using M06-2X with 6-311+G(d,p) basis set in water, DFT calculations of the molecular electrostatic potential (MEP), frontier molecular orbitals (FMOs), and the optimized geometry of the XRD values are compared with experimental values. All the synthesized novel isosteres molecules are investigated under molecular docking studies using MMP1 and MMP2 proteins, which showed all the molecules have the potential to heal pancreatic cancer. The most potent molecules among them are 3i and 3h due to their better docking scores. Furthermore, the molecules' pharmacokinetic (ADME) parameters have been observed to be effective in future biological evaluations of these compounds to be active.
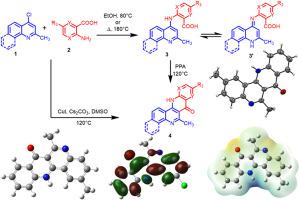
使用 CuI 催化剂合成新型萘啶类等效类似物:DFT 计算(FMO、MEP)、分子对接和 ADME 分析
一种通过 4-氯-2-甲基喹啉/4-氯-2-甲基苯并[h]喹啉与适当的邻氨基芳香族和杂芳香族羧酸之间的缩合反应合成新型苯并萘啶类和苯并萘啶类异构体的方法,采用溶剂(乙醇)/无溶剂(纯净)条件生成中间体,然后与 PPA 进行环化反应。与溶剂条件相比,在纯净(无溶剂)条件下,中间体产率略有增加。此外,在使用 CuI 催化剂的一锅合成法中,苯并萘啶和苯并萘啶的目标异构体的产率高于分步法。利用 M06-2X 和 6-311+G(d,p) 水基集对合成化合物进行了量子化学计算,并将分子静电势、前沿分子轨道和优化几何形状的 XRD 值与实验值进行了比较。利用 MMP1 和 MMP2 蛋白对所有合成的新型异构体分子进行了分子对接研究,结果表明所有分子都具有治疗胰腺癌的潜力。其中药效最强的分子是 3i 和 3h,因为它们的对接得分较高。此外,还观察到这些分子的药代动力学(ADME)参数,这对今后对这些化合物的活性进行生物学评价是有效的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


