Borosilicate bioactive glass synergizing low-dose antibiotic loaded implants to combat bacteria through ATP disruption and oxidative stress to sequentially achieve osseointegration
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Bone infection is a catastrophe in clinical orthopedics. Despite being the standard therapy for osteomyelitis, antibiotic-loaded polymethyl methacrylate (PMMA) cement has low efficiency against bacteria in biofilms. Furthermore, high-dose antibiotic-loaded implants carry risks of bacterial resistance, tissue toxicity, and impairment of local tissue healing. By incorporating borosilicate bioactive glass (BSG) into low-dose gentamicin sulfate (GS)-loaded PMMA cement, an intelligent strategy that synergistically eradicates bacteria and sequentially promotes osseointegration, was devised. Results showed that BSG did not compromises the handling properties of the cement, but actually endowed it with an ionic and alkaline microenvironment, thereby damaging the integrity of bacterial cell walls and membranes, inhibiting ATP synthesis by disrupting the respiratory chain in cell membranes and glycogen metabolism, and elevating reactive oxygen species (ROS) levels by weakening antioxidant components (peroxisomes and carotenoids). These antibacterial characteristics of BSG synergistically reinforced the effectiveness of GS, which was far below the actual clinical dosage, achieving efficient bacterial killing and biofilm clearance by binding to the 30S subunit of ribosomes. Furthermore, the released GS and the ionic and alkaline microenvironment from the implants fostered the osteogenic activity of hBMSCs in vitro and coordinately enhanced osseointegration in vivo. Collectively, this study underscores that BSG incorporation offers a promising strategy for reducing antibiotic dosage while simultaneously enhancing the antibacterial activity and osteogenesis of implants. This approach holds potential for resolving the conflict between bacterial resistance and bone infection.
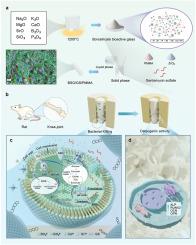
硼硅酸盐生物活性玻璃与低剂量抗生素植入体协同作用,通过 ATP 破坏和氧化应激抑制细菌,从而实现骨结合
骨感染是临床骨科的一大灾难。尽管抗生素载体聚甲基丙烯酸甲酯(PMMA)骨水泥是骨髓炎的标准疗法,但它对生物膜中细菌的抗菌效率很低。此外,高剂量抗生素植入物还存在细菌耐药性、组织毒性和影响局部组织愈合的风险。通过将硼硅酸盐生物活性玻璃(BSG)加入低剂量硫酸庆大霉素(GS)负载的 PMMA 骨水泥中,设计出了一种既能协同消灭细菌,又能依次促进骨结合的智能策略。结果表明,BSG 不仅不会损害骨水泥的处理特性,反而会使其具有离子和碱性微环境,从而破坏细菌细胞壁和细胞膜的完整性,通过破坏细胞膜和糖原代谢中的呼吸链来抑制 ATP 合成,并通过削弱抗氧化成分(过氧化物酶体和类胡萝卜素)来提高活性氧(ROS)水平。BSG 的这些抗菌特性协同加强了 GS 的效力,而 GS 的用量远低于实际临床用量,通过与核糖体 30S 亚基结合,实现了高效杀灭细菌和清除生物膜的目的。此外,植入物释放的 GS 和离子碱性微环境在体外促进了 hBMSCs 的成骨活性,在体内协调增强了骨结合。总之,这项研究强调了 BSG 的加入为减少抗生素用量,同时提高植入物的抗菌活性和成骨能力提供了一种可行的策略。这种方法有望解决细菌耐药性和骨感染之间的矛盾。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


