Enhanced desalination performance of flow capacitive deionization with the addition of conductive polymer in redox couples and activated carbon
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Freshwater scarcity is a critical global issue and desalination of brackish water and seawater technologies are regarded as effective solution to mitigate the increasing severity of water shortage. Flow-electrode capacitive deionization (FCDI) is an emerging electrochemical desalination technology capable of continuous deionization behavior. However, reducing energy consumption and enhancing desalination rate are now markedly needed for its advancement. Herein, we propose a FCDI system with energy consumption as low as 88.08 kJ mol−1 and a desalting rate of 1.75 μg cm−2 s−1. This is achieved by using flow electrodes containing 0.03125 wt% conductive polymer, 5 wt% activated carbon/carbon black and 80 mM/80 mM ferricyanide/ferrocyanide at a current density 3 mA cm−2 (3.36 mA current for a 1.12 cm2 active area). We further investigate the effects of polymer content, redox pair content, salt content and current densities on desalination performance. Seawater with a conductivity of 52.78 mS cm−1 was successfully desalinated to 0.50 mS cm−1 in continuous process. This research provides a promising approach to enhance FCDI system by achieving a low energy consumption and high salt removal rate, representing a significant advancement in continuous electrochemical desalination technology.
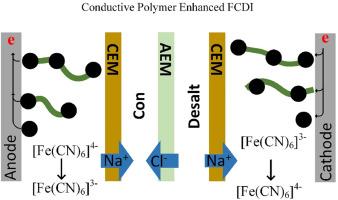
在氧化还原耦合剂和活性炭中添加导电聚合物,提高流动电容式去离子法的海水淡化性能
淡水匮乏是一个严重的全球性问题,而苦咸水和海水淡化技术被认为是缓解日益严重的水资源短缺问题的有效解决方案。流动电极电容式去离子(FCDI)是一种新兴的电化学海水淡化技术,能够连续进行去离子操作。然而,降低能耗和提高脱盐率是该技术发展的当务之急。在此,我们提出了一种能耗低至 88.08 kJ mol-1、脱盐率为 1.75 μg cm-2 s-1 的 FCDI 系统。这是通过使用含有 0.03125 wt% 导电聚合物、5 wt% 活性炭/炭黑和 80 mM/80 mM 三氯化铁/铁氰化物的流动电极在 3 mA cm-2 的电流密度下实现的(1.12 cm2 活性面积的电流为 3.36 mA)。我们进一步研究了聚合物含量、氧化还原对含量、盐含量和电流密度对海水淡化性能的影响。在连续过程中,电导率为 52.78 mS cm-1 的海水成功脱盐至 0.50 mS cm-1。这项研究通过实现低能耗和高盐去除率,为增强 FCDI 系统提供了一种可行的方法,标志着连续电化学海水淡化技术的重大进步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


