Multi-synergy enabling Ni-doped MoS2@N-doped carbon composite as versatile catalysts toward hydrogen production and photovoltaics
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Construction of efficient non-precious catalyst with desired chemical composition and well-defined nanostructure is of great essential to enhance the kinetics of hydrogen evolution reaction (HER) and triiodide (I3−) reduction reaction (IRR), which is conducive to promote the development of green hydrogen production and obtain impressive photovoltaic performance of dye-sensitized solar cells (DSSCs). Herein, ZIF-8 derived N-doped carbon (NC) wrapped with two-dimensional Ni-doped MoS2 nanosheets (Ni–MoS2@NC) are elaborately designed. The catalytic activity of Ni–MoS2@NC for HER and IRR are significantly improved by modifying electronic structure through heteroatom doping. The optimized Ni–MoS2@NC has lower overpotential of 122 mV at 10 mA cm−2 in comparison with counterpart. Meanwhile, the power conversion efficiency (PCE) of DSSCs based on Ni–MoS2@NC CE catalyst is comparable to that of Pt. Density functional theory (DFT) calculation is used to unveil the mechanism of Ni–MoS2@NC for alkaline HER and IRR, namely, the multi-synergy of various sites endows Ni–MoS2@NC with appropriate Gibbs free energy for H∗ adsorption and water dissociation energy, while the top and interfacial S sites of Ni–MoS2@NC are responsible for the adsorption and activation of I3−. Our work provides a feasible route to design efficient catalysts in the field of energy conversion and understand mechanism.
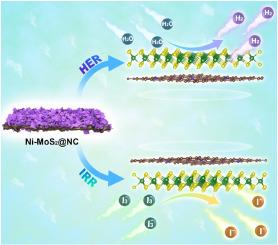
掺杂镍的 MoS2@N 掺杂碳复合材料作为多功能催化剂,可用于制氢和光伏技术
构建具有所需化学成分和明确纳米结构的高效非贵金属催化剂对于提高氢进化反应(HER)和三碘化物(I3-)还原反应(IRR)的动力学至关重要,这有利于促进绿色制氢的发展,并使染料敏化太阳能电池(DSSC)获得令人瞩目的光电性能。本文精心设计了由 ZIF-8 衍生的掺杂 N 的碳(NC)包裹二维掺杂 Ni- 的 MoS2 纳米片(Ni-MoS2@NC)。通过掺杂杂原子改变电子结构,Ni-MoS2@NC 对 HER 和 IRR 的催化活性得到显著提高。与同类催化剂相比,优化后的 Ni-MoS2@NC 在 10 mA cm-2 时的过电位更低,仅为 122 mV。同时,基于 Ni-MoS2@NC CE 催化剂的 DSSC 的功率转换效率(PCE)与铂相当。密度泛函理论(DFT)计算揭示了 Ni-MoS2@NC 在碱性 HER 和 IRR 中的作用机理,即不同位点的多重协同作用赋予了 Ni-MoS2@NC 适当的 H∗ 吸附吉布斯自由能和水解离能,而 Ni-MoS2@NC 的顶部和界面 S 位点则负责 I3- 的吸附和活化。我们的工作为设计能量转换领域的高效催化剂和了解其机理提供了一条可行的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


