Environment-dependent tribochemical reaction and wear mechanisms of Diamond-like carbon: A reactive molecular dynamics study
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
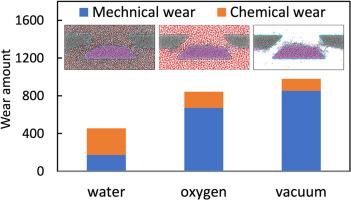
Diamond-like carbon (DLC) is widely utilized in various fields as a promising solid lubricating material. However, the lubricity and wear behaviors of DLC are highly sensitive to the environment, and the relevant mechanisms are hidden by complex tribochemical reactions at the friction interface. In this study, we performed reactive molecular dynamics (RMD) simulations of DLC to clarify the wear mechanisms in various environments (vacuum, water, and oxygen environments). Two types of tribochemical reaction-induced wear were observed: chemical wear induced by the triboemission of CxHy and CxHyOz compounds and mechanical wear induced by the formation of interfacial C–C bonds. Moreover, the results revealed that the environment affects the tribological behavior of DLC primarily through its impact on the surface chemical state, that is, the quantity and type of surface terminations. In terms of the number of surface terminations, the more surface terminations there are, the more chemical wear and less mechanical wear they cause. In particular, oxygen-containing terminations (e.g., C–OH and C=O) are more resistant than H terminations to interfacial bond formation and mechanical wear. The present work provides important insights into the wear mechanisms of DLC, aiding in the reduction of DLC wear by controlling the environment.
类金刚石碳与环境相关的摩擦化学反应和磨损机理:反应分子动力学研究
类金刚石碳(DLC)作为一种前景广阔的固体润滑材料被广泛应用于各个领域。然而,类金刚石碳的润滑性和磨损行为对环境高度敏感,相关机理被摩擦界面上复杂的摩擦化学反应所掩盖。在这项研究中,我们对 DLC 进行了反应分子动力学(RMD)模拟,以阐明其在各种环境(真空、水和氧气环境)中的磨损机制。我们观察到两种摩擦化学反应引起的磨损:由 CxHy 和 CxHyOz 化合物的三发射引起的化学磨损和由界面 C-C 键的形成引起的机械磨损。此外,研究结果表明,环境对 DLC 摩擦学行为的影响主要是通过其对表面化学状态的影响,即表面端接的数量和类型。就表面端接的数量而言,表面端接越多,化学磨损越大,机械磨损越小。尤其是含氧端接(如 C-OH 和 C=O)比 H 端接更能抵抗界面键的形成和机械磨损。本研究为了解 DLC 的磨损机理提供了重要依据,有助于通过控制环境来减少 DLC 磨损。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


