From tradition to evidence-base: Leveraging TCM human use experience in modern drug development
Pharmacological Research - Modern Chinese Medicine
Pub Date : 2024-10-22
DOI:10.1016/j.prmcm.2024.100535
引用次数: 0
Abstract
Introduction
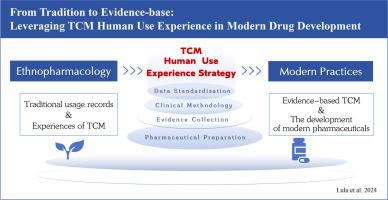
Traditional Chinese medicines (TCM) have been used in China for hundreds, and in some cases, thousands of years, accumulating substantial clinical experience known as "TCM Human Use Experience." These medicines are also widely used in modern medical institutions, presenting the challenge of leveraging this extensive experience and applying modern evidence-based medicine and pharmaceutical methods for the scientific research and development of new drugs from ethnic medicines.
Methods
A literature and regulatory search was conducted using PubMed, Web of Science, CNKI, and relevant national databases (CDE, FDA, PMDA, EMA) to collect peer-reviewed studies and policies on TCM human use experience and its integration into modern drug development. In total, 112 articles and regulations were reviewed, with 12 excluded due to irrelevance to empirical TCM applications.
Results
Our review identifies the methodological challenges and innovations necessary for integrating TCM into the modern medical landscape. We propose a systematic approach for the collection and analysis of TCM clinical data, including the establishment of a standardized database for TCM human use experience, the translation of TCM concepts into modern medical practices, and ensuring efficacy, safety, and regulatory compliance through rigorous data methodologies.
Discussion
Our findings advocate for a balanced approach that respects the rich heritage of TCM while embracing contemporary scientific and clinical standards. This integration has the potential to enrich global healthcare with diverse, evidence-based treatment options, significantly contributing to the broader acceptance and integration of TCM into global healthcare systems.
从传统到证据基础:在现代药物开发中利用中医药的人体使用经验
导言传统中药(TCM)在中国已经使用了数百年,有时甚至数千年,积累了丰富的临床经验,被称为 "中药人体使用经验"。方法利用 PubMed、Web of Science、CNKI 和相关国家数据库(CDE、FDA、PMDA、EMA)进行文献和法规检索,以收集有关中医药人体使用经验及其与现代药物开发相结合的同行评议研究和政策。结果我们的研究发现了将中药融入现代医学所需的方法学挑战和创新。我们提出了收集和分析中医临床数据的系统方法,包括建立标准化的中医人体使用经验数据库,将中医概念转化为现代医疗实践,以及通过严格的数据方法确保疗效、安全性和合规性。讨论我们的研究结果主张采取一种平衡的方法,既尊重中医的丰富遗产,又接受现代科学和临床标准。这种整合有可能为全球医疗保健提供多样化的循证治疗方案,极大地促进中医药被更广泛地接受并融入全球医疗保健体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


