The mechanism of selective separation of stibnite and arsenopyrite by Cu2+ coordination assembly KBX collector
IF 4.9
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The similar physicochemical properties of stibnite and arsenopyrite resulted in the difficult separation by traditional collectors in flotation. Hence, this study utilized Cu2+ and potassium butyl xanthate (KBX) coordinate assembly to form a novel Cu-KBX complex collector, investigating its properties, conformation, and its role in flotation separation of stibnite and arsenopyrite, as well as its diverse adsorption behavior on mineral surfaces. The stable Cu-KBX complex solution was formed when the molar ratio of Cu2+ to KBX was 1:2, exhibiting a network-like structure at that time. The micro-flotation results showed that at pH 5, the grade of Sb in the concentrate was as high as 61.08 %, with As content at only 4.65 %, enabling effective separation of stibnite and arsenopyrite. Furthermore, the Cu-KBX complex exhibited a reticulated structure at the stibnite surface, while it adhered in a granular fashion on the arsenopyrite surface. FTIR analysis confirmed stronger chemisorption of Cu-KBX onto stibnite compared to arsenopyrite. XPS results indicated that Cu-S was the main collecting component. However, a weak Fe(II)-S substance was found on the arsenopyrite interface, likely due to Fe3+ oxidizing in the slurry with the Cu-KBX complex. Therefore, this disruption of the Cu-KBX complex structure by Fe3+ in arsenopyrite sharply reduced its adsorption on arsenopyrite, enhancing the selective separation of stibnite and arsenopyrite.
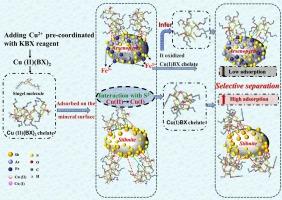
Cu2+配位组装KBX收集器选择性分离锡黄铁矿和砷黄铁矿的机理
由于闪锌矿和砷黄铁矿具有相似的物理化学性质,因此在浮选过程中很难用传统的捕收剂进行分离。因此,本研究利用 Cu2+ 与黄原酸丁酯钾(KBX)配位组装形成新型 Cu-KBX 复合捕收剂,研究其性质、构象及其在浮选分离锡黄铁矿和砷黄铁矿中的作用,以及在矿物表面的多种吸附行为。当 Cu2+ 与 KBX 的摩尔比为 1:2 时,形成了稳定的 Cu-KBX 复合物溶液,并呈现出网状结构。微浮选结果表明,在 pH 值为 5 时,精矿中锑的品位高达 61.08%,而砷的含量仅为 4.65%,从而有效地分离了锡黄铁矿和砷黄铁矿。此外,铜-KBX 复合物在锡黄铁矿表面呈现网状结构,而在砷黄铁矿表面则呈颗粒状附着。傅立叶变换红外分析证实,与黄铜矿相比,Cu-KBX 在闪锌矿上的化学吸附作用更强。XPS 结果表明,Cu-S 是主要的收集成分。然而,在砷黄铁矿界面上发现了微弱的 Fe(II)-S 物质,这可能是由于泥浆中的 Fe3+ 与 Cu-KBX 复合物一起氧化所致。因此,砷黄铁矿中的 Fe3+ 破坏了 Cu-KBX 复合物的结构,大大降低了其在砷黄铁矿上的吸附力,增强了闪锌矿和砷黄铁矿的选择性分离。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


