A new route for the synthesis of substituted benzo [1,3,4] oxadiazine derivatives via copper-catalyzed N-arylation-cyclization of hydrazonoyl chlorides and 2-iodophenol
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this research, a rapid and direct approach for the synthesis of benzo [1,3,4] oxadiazine derivatives via a copper-catalyzed intermolecular N-arylation of 2-iodophenol and hydrozonoyl chloride is described. The reaction occurs rapidly at room temperature, making it convenient and time-saving. No complex ligands or special equipment are required. The use of simple and readily available raw materials, without column chromatography, mild catalytic copper reaction conditions, and good yield (71–93 %), are notable features of this protocol. The [1,3,4] oxadiazine moiety is often found in drugs and other bioactive molecules.
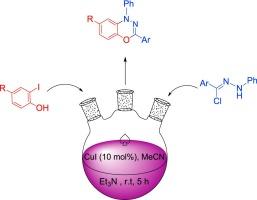
通过铜催化肼酰氯和 2-碘苯酚的 N-芳基化-环化合成取代的苯并 [1,3,4] 恶二嗪衍生物的新途径
本研究介绍了一种通过铜催化 2-碘苯酚和氢宗酰氯的分子间 N-芳基化反应合成苯并 [1,3,4] 恶二嗪衍生物的快速直接方法。反应在室温下快速进行,既方便又省时。不需要复杂的配体或特殊设备。使用简单易得的原料、无需柱层析、温和的铜催化反应条件和良好的收率(71-93%)是该方法的显著特点。[1,3,4]恶二嗪分子通常存在于药物和其他生物活性分子中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


