Scalable synthesis of sulfonium and selenonium peptides for selective methyllysine reader crosslinking
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Lysine methylation readers are an important class of proteins that bind site-specifically methylated proteins for downstream regulation. Chemical probes that crosslink lysine methylation readers are highly desired to investigate the proteins from cellular samples. We recently reported NleS+me2 (norleucine-ε-dimethylsulfonium) as dimethyllysine mimic selectively crosslinks corresponding methyllysine readers. Although the sulfonium tools exhibited great promise, the synthetic availability may limit broad applications. In order to incorporate the unnatural amino acid, l-Fmoc-NleSme-OH (Fmoc: fluorenyl methoxycarbonyl) was synthesized as an important building block for solid-phase peptide synthesis (SPPS) in the previous synthetic route. It took six steps with resolution of racemic mixture and radical mediated thiol-ene reaction. As a result, the synthesis was not scalable with only 4.4 % overall yield. Here we report a much-improved synthesis method so that diverse NleS+me2 peptides could be readily prepared. In addition, the new method enables preparation of selenonium peptides for methyllysine reader crosslinking. We thus believe this synthetic method will be widely used to prepare sulfonium and selenonium probes for site-selective crosslinking.
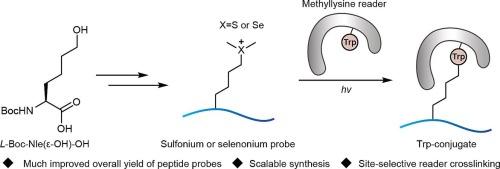
用于选择性甲赖氨酸读取器交联的锍肽和硒肽的规模化合成
赖氨酸甲基化读取器是一类重要的蛋白质,它能结合特定位点的甲基化蛋白质进行下游调控。交联赖氨酸甲基化读取器的化学探针在研究细胞样本中的蛋白质时非常有用。我们最近报道了 NleS+me2(norleucine-ε-dimethylsulfonium)作为二甲基赖氨酸模拟物选择性地交联相应的甲基赖氨酸阅读器。尽管锍工具显示出巨大的前景,但其合成可用性可能会限制其广泛应用。为了加入非天然氨基酸,在之前的合成路线中,l-Fmoc-NleSme-OH(Fmoc:芴甲氧羰基)作为固相肽合成(SPPS)的重要构件被合成出来。它需要六个步骤,包括外消旋混合物的解析和自由基介导的硫醇-烯反应。因此,这种合成方法无法推广,总产率仅为 4.4%。在此,我们报告了一种经过大幅改进的合成方法,从而可以轻松制备出多种 NleS+me2 肽。此外,新方法还能制备用于甲赖氨酸读取器交联的硒肽。因此,我们相信这种合成方法将被广泛用于制备用于位点选择性交联的锍和硒探针。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


