Melting temperature and elastic constants of disordered nonstoichiometric cubic TiCy, ZrCy and HfCy carbides
IF 4.2
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
International Journal of Refractory Metals & Hard Materials
Pub Date : 2024-10-18
DOI:10.1016/j.ijrmhm.2024.106920
引用次数: 0
Abstract
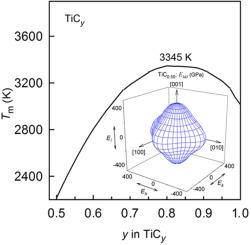
Based on the analysis of phase diagrams of carbide-forming systems M–C (M = Ti, Zr, Hf), an empirical relationship is proposed between the elastic stiffness constants cij of nonstoichiometric cubic carbides of titanium, zirconium and hafnium and their melting temperature. The dependences of the melting temperatures of nonstoichiometric cubic carbides TiCy, ZrCy and HfCy on their composition in homogeneity regions are calculated using the elastic stiffness constants c11(y) and c44(y) of these carbides. The calculated maximum melting temperatures are observed for carbides ∼TiC0.80, ∼ZrC0.82 and ∼ HfC0.94–0.95 and are equal to 3345, 3708 and 4192 K, respectively. There is a qualitative correlation between the concentration dependences of the melting temperatures Tm(y) of TiCy, ZrCy and HfCy carbides and the anisotropy of the elastic properties of these carbides.
无序非均匀立方 TiCy、ZrCy 和 HfCy 碳化物的熔化温度和弹性常数
根据对碳化物形成体系 M-C(M = Ti、Zr、Hf)相图的分析,提出了钛、锆和铪的非化学计量立方碳化物的弹性刚度常数 cij 与它们的熔化温度之间的经验关系。利用这些碳化物的弹性刚度常数 c11(y) 和 c44(y),计算了非均质立方碳化物 TiCy、ZrCy 和 HfCy 在均质区的熔化温度与其成分的关系。碳化物∼TiC0.80、∼ZrC0.82 和∼HfC0.94-0.95 的计算最高熔化温度分别为 3345、3708 和 4192 K。TiCy、ZrCy 和 HfCy 碳化物的熔化温度 Tm(y) 的浓度依赖性与这些碳化物弹性特性的各向异性之间存在定性关联。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
13.90%
发文量
236
审稿时长
35 days
期刊介绍:
The International Journal of Refractory Metals and Hard Materials (IJRMHM) publishes original research articles concerned with all aspects of refractory metals and hard materials. Refractory metals are defined as metals with melting points higher than 1800 °C. These are tungsten, molybdenum, chromium, tantalum, niobium, hafnium, and rhenium, as well as many compounds and alloys based thereupon. Hard materials that are included in the scope of this journal are defined as materials with hardness values higher than 1000 kg/mm2, primarily intended for applications as manufacturing tools or wear resistant components in mechanical systems. Thus they encompass carbides, nitrides and borides of metals, and related compounds. A special focus of this journal is put on the family of hardmetals, which is also known as cemented tungsten carbide, and cermets which are based on titanium carbide and carbonitrides with or without a metal binder. Ceramics and superhard materials including diamond and cubic boron nitride may also be accepted provided the subject material is presented as hard materials as defined above.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


