Enhanced healing of critical-sized bone defects using degradable scaffolds with tailored composition through immunomodulation and angiogenesis
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The impact of orthopedic scaffolds on bone defect healing, particularly the late-stage bone remodeling process, is pivotal for the therapeutic outcome. This study applies fadditively manufactured scaffolds composed of hydroxyapatite-doped poly(lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(lactide-co-glycolide) (HA-PELGA) with varying properties to treat rat calvarial defects, elucidating their significant role in bone remodeling by modulating physiological responses. We engineered two scaffolds with different polylactic acid (PLA) to polyglycolic acid (PGA) ratio (9/1 and 18/1) to vary in hydrophobicity, degradation rate, mechanical properties, and structural stability. These variations influenced physiological responses, including osteogenesis, angiogenesis, and immune reactions, thereby guiding bone remodeling. Our findings show that the HA-PELGA(18/1) scaffold, with a slower degradation rate, supported bulk bone formation due to a stable microenvironment. Conversely, the HA-PELGA(9/1) scaffold, with a faster degradation rate and more active interfaces, facilitated the formation of a thin bone layer and higher bone infiltration. This study demonstrates these degradable scaffolds help to promote bone healing and reveals how scaffold properties influence the bone remodeling process, offering a potential strategy to optimize scaffold design aiming at late-stage bone defect healing.
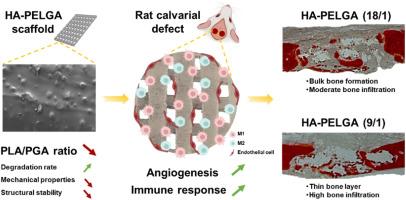
使用具有定制成分的可降解支架,通过免疫调节和血管生成促进临界大小骨缺损的愈合
骨科支架对骨缺损愈合,尤其是后期骨重塑过程的影响对治疗效果至关重要。本研究采用掺杂羟基磷灰石的聚(乳聚乙二醇)-b-聚(乙二醇)-b-聚(乳聚乙二醇)(HA-PELGA)快速制造不同性质的支架来治疗大鼠腓骨缺损,通过调节生理反应来阐明其在骨重塑中的重要作用。我们设计了两种不同聚乳酸(PLA)与聚乙二醇酸(PGA)比例(9/1 和 18/1)的支架,以改变疏水性、降解率、机械性能和结构稳定性。这些变化会影响生理反应,包括骨生成、血管生成和免疫反应,从而指导骨重塑。我们的研究结果表明,HA-PELGA(18/1)支架降解速度较慢,但由于微环境稳定,可支持大块骨形成。相反,HA-PELGA(9/1)支架降解速度更快,界面更活跃,有利于形成薄骨层和更高的骨浸润。这项研究表明,这些可降解支架有助于促进骨愈合,并揭示了支架特性如何影响骨重塑过程,为优化支架设计以实现晚期骨缺损愈合提供了一种潜在的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


