3D-printed manganese dioxide incorporated scaffold promotes osteogenic-angiogenic coupling for refractory bone defect by remodeling osteo-regenerative microenvironment
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The treatment of refractory bone defects is a major clinical challenge, especially in steroid-associated osteonecrosis (SAON), which is characterized by insufficient osteogenesis and angiogenesis. Herin, a microenvironment responsiveness scaffold composed of poly-L-lactide (PLLA), and manganese dioxide (MnO2) nanoparticles is designed to enhance bone regeneration by scavenging endogenous reactive oxygen species (ROS) and modulating immune microenvironment in situ. A catalase-like catalytic reaction between MnO2 and endogenous hydrogen peroxide (H2O2) generated at the bone defect area, which typically becomes acidic and ROS-rich, triggers on-demand release of oxygen and Mn2+, significantly ameliorating inflammatory response by promoting M2-type polarization of macrophages, reprograming osteoimmune microenvironment conducive to angiogenesis and osteogenesis. Furthermore, the fundamental mechanisms were explored through transcriptome sequencing analysis, revealing that PLLA/MnO2 scaffolds (PMns) promote osteogenic differentiation by upregulating the TGF-β/Smad signaling pathway in human bone marrow mesenchymal stem cells (hBMSCs). Overall, the PMns exhibit superior immunomodulatory, excellent osteogenic-angiogenic properties and promising candidates as bone graft substitutes for therapy clinical refractory bone defects.
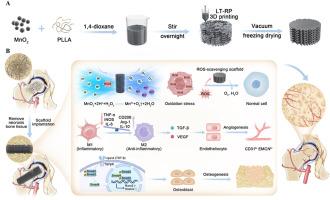
融入二氧化锰的三维打印支架通过重塑骨再生微环境,促进难治性骨缺损的骨生成-血管生成耦合
难治性骨缺损的治疗是一项重大的临床挑战,尤其是类固醇相关性骨坏死(SAON),其特点是骨生成和血管生成不足。Herin是一种由聚乳酸(PLLA)和二氧化锰(MnO2)纳米颗粒组成的微环境响应性支架,旨在通过清除内源性活性氧(ROS)和原位调节免疫微环境来促进骨再生。骨缺损区域通常呈酸性且富含 ROS,MnO2 与该区域产生的内源性过氧化氢(H2O2)发生类似催化酶的催化反应,按需释放氧气和 Mn2+,通过促进巨噬细胞的 M2 型极化显著改善炎症反应,重编程有利于血管生成和骨生成的骨免疫微环境。此外,通过转录组测序分析探索了其基本机制,发现聚乳酸/二氧化锰支架(PMns)通过上调人骨髓间充质干细胞(hBMSCs)的TGF-β/Smad信号通路促进成骨分化。总之,PMns 具有卓越的免疫调节和成骨-血管生成特性,有望成为治疗临床难治性骨缺损的骨移植替代物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


