The response mechanism of alkalophilic Nitzschia sp. NW129 to low alkalinity-A study combining physiological and transcriptional analysis
IF 4.6
2区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
Algal Research-Biomass Biofuels and Bioproducts
Pub Date : 2024-10-18
DOI:10.1016/j.algal.2024.103748
引用次数: 0
Abstract
Global aquatic acidification significantly threatens alkaline lake ecosystems. The mechanisms by which alkaliphilic microalgae, the key producers in these ecosystems, respond to reduced environmental alkalinity remain poorly understood. Here, we investigated the responses of alkalophilic Nitzschia sp. NW129 to low alkalinity (pH 9.2) through integrated physiological-biochemical and transcriptomic analyses. Relative to the control (pH 11.5), we observed a 60.1 % decrease in polysaccharide content, while total lipids and proteins increased by 1.74-fold and 2-fold, respectively. Transcriptome analysis revealed up-regulation of genes encoding carbonic anhydrase (CA) and malic enzyme (ME), along with those involved in glycolysis and fatty acid (FA) synthesis, compensating for carbon supply and shifting carbon flux from carbohydrate synthesis to lipid accumulation. Enhanced expression of TCA cycle genes and those encoding F-ATP synthase and inorganic pyrophosphatase (PPase) provided sufficient energy for cellular homeostasis, further facilitated by the up-regulated expression of ATP-dependent V-ATPase and ABC transporter genes. Temporal analysis revealed that the expression of genes involved in protein synthesis pathways was up-regulated on days 1 and 4 but notably down-regulated on day 2, suggesting protein degradation at this time to balance energy supply for adaptation. Despite these coping shifts, impairments in photosynthetic energy dissipation and electron transport, along with transcriptional changes including down-regulating cell cycle and inducing apoptotic pathways, ultimately caused a substantial reduction in biomass. These findings provide a basic understanding of the response mechanisms of alkalophilic microalgae to low alkalinity stress, which should aid to develop strategies to improve microalgal tolerance against acidification.
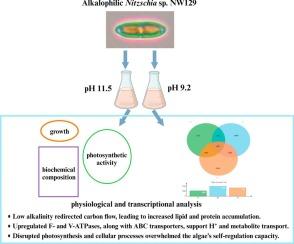
嗜碱尼茨藻 NW129 对低碱度的响应机制--生理和转录分析相结合的研究
全球水生酸化严重威胁着碱性湖泊生态系统。嗜碱微藻是这些生态系统中的主要生产者,它们对环境碱度降低的反应机制仍然知之甚少。在此,我们通过生理生化和转录组学综合分析,研究了嗜碱性 Nitzschia sp.与对照组(pH 11.5)相比,我们观察到多糖含量减少了 60.1%,而总脂类和蛋白质分别增加了 1.74 倍和 2 倍。转录组分析表明,编码碳酸酐酶(CA)和苹果酸酶(ME)的基因以及参与糖酵解和脂肪酸(FA)合成的基因上调,补偿了碳供应并将碳通量从碳水化合物合成转移到脂质积累。TCA循环基因以及编码F-ATP合成酶和无机焦磷酸酶(PP酶)的基因表达增强,为细胞平衡提供了充足的能量,依赖ATP的V-ATP酶和ABC转运体基因的表达上调进一步促进了细胞平衡。时间分析表明,蛋白质合成途径相关基因的表达在第1天和第4天上调,但在第2天明显下调,表明此时蛋白质降解以平衡适应所需的能量供应。尽管出现了这些应对转变,但光合能量耗散和电子传递的损伤,以及包括下调细胞周期和诱导细胞凋亡途径在内的转录变化,最终导致生物量大幅减少。这些发现为了解嗜碱微藻对低碱度胁迫的响应机制提供了一个基本认识,有助于制定策略来提高微藻对酸化的耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Algal Research-Biomass Biofuels and Bioproducts
BIOTECHNOLOGY & APPLIED MICROBIOLOGY-
CiteScore
9.40
自引率
7.80%
发文量
332
期刊介绍:
Algal Research is an international phycology journal covering all areas of emerging technologies in algae biology, biomass production, cultivation, harvesting, extraction, bioproducts, biorefinery, engineering, and econometrics. Algae is defined to include cyanobacteria, microalgae, and protists and symbionts of interest in biotechnology. The journal publishes original research and reviews for the following scope: algal biology, including but not exclusive to: phylogeny, biodiversity, molecular traits, metabolic regulation, and genetic engineering, algal cultivation, e.g. phototrophic systems, heterotrophic systems, and mixotrophic systems, algal harvesting and extraction systems, biotechnology to convert algal biomass and components into biofuels and bioproducts, e.g., nutraceuticals, pharmaceuticals, animal feed, plastics, etc. algal products and their economic assessment
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


