Preventing postsurgical colorectal cancer relapse: A hemostatic hydrogel loaded with METTL3 inhibitor for CAR-NK cell therapy
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Colorectal cancer (CRC) recurrence post-surgery remains a major challenge. While Chimeric Antigen Receptor (CAR)-engineered natural killer (NK) cells hold immense therapeutic potential, their intratumoral infiltration ability remains limited, hampering efficacy. Building upon prior research suggesting that chemokines like C-X-C motif chemokine ligand 9 (CXCL9) and C-X-C motif chemokine ligand 10 (CXCL10) recruit CAR-NK cells, we hypothesized that tumor cell m6A methylation, regulated by Methyltransferase-like 3 (METTL3), influences chemokine secretion. This study aims to elucidate the underlying mechanisms and improve METTL3 inhibition efficiency. We designed an adhesive hemostasis hydrogel loaded with STM2457, a METTL3 inhibitor, aimed at sustained release in the acidic tumor microenvironment. In vitro, the hydrogel promoted CAR-NK cell recruitment and tumor killing via sustained METTL3 inhibition. The hydrogel's Schiff base bonds further enabled intestinal adhesion and hemostasis in an incomplete tumor resection model of CRC. Combining the hydrogel with CAR-NK cell therapy significantly reduced CRC recurrence in vivo. Overall, our study reveals the crucial role of METTL3 in CRC recurrence and proposes a promising, multimodal strategy using STM2457-loaded hydrogel and CAR-NK cells for enhanced therapeutic efficacy.
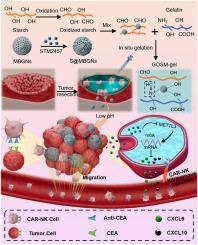
预防手术后结直肠癌复发:用于 CAR-NK 细胞疗法的装载有 METTL3 抑制剂的止血水凝胶
结直肠癌(CRC)术后复发仍是一大挑战。虽然嵌合抗原受体(CAR)工程化的自然杀伤(NK)细胞具有巨大的治疗潜力,但其瘤内浸润能力仍然有限,影响了疗效。之前的研究表明,C-X-C motif趋化因子配体9(CXCL9)和C-X-C motif趋化因子配体10(CXCL10)等趋化因子能招募CAR-NK细胞,在此基础上,我们推测肿瘤细胞m6A甲基化受类似甲基转移酶3(METTL3)的调控,会影响趋化因子的分泌。本研究旨在阐明其潜在机制并提高 METTL3 的抑制效率。我们设计了一种粘附止血水凝胶,其中装载了 METTL3 抑制剂 STM2457,旨在酸性肿瘤微环境中持续释放。在体外,该水凝胶通过持续抑制 METTL3 促进了 CAR-NK 细胞的募集和肿瘤杀伤。在不完全肿瘤切除的 CRC 模型中,水凝胶的 Schiff 碱键进一步实现了肠道粘附和止血。将水凝胶与 CAR-NK 细胞疗法相结合,可显著降低 CRC 在体内的复发率。总之,我们的研究揭示了 METTL3 在 CRC 复发中的关键作用,并提出了一种利用 STM2457 水凝胶和 CAR-NK 细胞提高疗效的前景广阔的多模式策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


