Scaffolds functionalized with matrix metalloproteinase-responsive release of miRNA for synergistic magnetic hyperthermia and sensitizing chemotherapy of drug-tolerant breast cancer
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Combining hyperthermia and chemotherapy for maximum anticancer efficacy remains a challenge because drug-tolerant cancer cells often evade this synergistic treatment due to drug resistance and asynchronous drug release. In this study, multifunctional scaffolds were designed to efficiently treat drug-tolerant breast cancer by improving the sensitization of breast cancer cells and synchronizing anticancer drug release with magnetic hyperthermia. The scaffolds contained microRNA-encapsulated matrix metalloproteinase-cleavable liposomes, doxorubicin-encapsulated thermoresponsive liposomes and Fe3O4 nanoparticles. The scaffolds could release microRNA specifically to improve the sensitization of breast cancer cells to anticancer drugs. The scaffolds also showed excellent hyperthermia effects under alternating magnetic field irradiation. Moreover, doxorubicin release was synchronized with magnetic hyperthermia. In vitro and in vivo studies demonstrated that the scaffolds effectively reduced drug resistance and eliminated doxorubicin-tolerant MDA-MB-231 cells through the synergistic effect of magnetic hyperthermia and sensitizing chemotherapy. Additionally, the scaffolds could support the proliferation and adipogenic differentiation of stem cells for adipose tissue regeneration after killing cancer cells at a late therapeutic stage. These composite scaffolds offer an innovative strategy for treating breast cancer, with synergistic anticancer effects and regenerative functions.
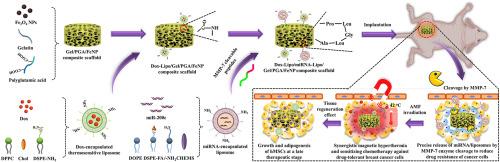
基质金属蛋白酶响应性释放 miRNA 的功能化支架,用于协同磁性热疗和药物耐受性乳腺癌的增敏化疗
由于耐药性和药物释放不同步,耐药癌细胞往往会逃避这种协同治疗,因此将热疗与化疗相结合以获得最大抗癌疗效仍然是一项挑战。本研究设计了多功能支架,通过提高乳腺癌细胞的敏感性,使抗癌药物释放与磁热疗同步,从而有效治疗耐药性乳腺癌。该支架包含微RNA包裹的基质金属蛋白酶可清除脂质体、多柔比星包裹的热容脂质体和Fe3O4纳米颗粒。这些支架可以特异性地释放 microRNA,从而提高乳腺癌细胞对抗癌药物的敏感性。在交变磁场辐照下,支架还表现出卓越的热效应。此外,多柔比星的释放与磁热效应同步。体外和体内研究表明,该支架通过磁热效应和增敏化疗的协同作用,有效降低了耐药性,并消除了耐多柔比星的 MDA-MB-231 细胞。此外,在晚期治疗阶段杀死癌细胞后,这种支架还能支持干细胞的增殖和成脂分化,促进脂肪组织再生。这些复合支架为治疗乳腺癌提供了一种创新策略,具有协同抗癌效果和再生功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


