Alloying effects on the reactivity of Pd are ensemble dominated
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this paper the geometric (“ensemble”) and electronic (“ligand”) effects of alloying on surface reactivity and catalysis are considered. The effect of alloying on the behaviour of Pd, both in single crystal form, and as a nanoparticulate catalyst is discussed. The first case concerns Pd alloyed with Cu, and here the reactivity with formic acid and ethanol is modified by the presence of Cu. However, both Cu and Pd maintain their elemental integrity for the reactions, and it is shown that the main alloying effect is one of dilution of Pd atoms, rather than by global electronic factors such as d-band shifting and filling. Similarly, when Pd is alloyed with Au, then the adsorption characteristics (sticking probability and uptake) for CO, O2, ethene and acetaldehyde are dominated by changes in the surface arrangement of the two atoms. Au mainly acts as an adsorption blocker, but to different degrees depending upon the nature of the adsorbing molecule and its demand for particular ensemble sizes. Finally, nanoparticulate Pd is considered, and the effect of alloying on high pressure methanol synthesis from CO2 and H2 is outlined. Pd on its own is not very selective, instead it mainly produces CO and methane. However, by supporting on oxides such as ZnO, Ga2O3 and In2O3 and by reducing in hydrogen, the Pd forms alloys, which then results in high selectivity to methanol. Again, this is ascribed to the dilution of the Pd ensembles at the surface, which are the cause of methane production.
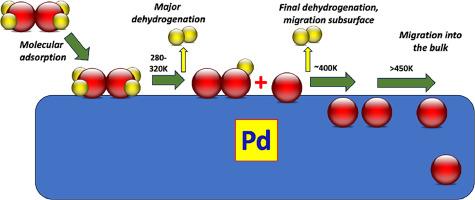
合金对钯反应性的影响以集合效应为主
本文探讨了合金化对表面反应性和催化作用的几何("集合")和电子("配体")效应。本文讨论了合金化对单晶钯和纳米颗粒催化剂行为的影响。第一种情况涉及与铜合金化的钯,铜的存在改变了钯与甲酸和乙醇的反应性。然而,铜和钯在反应中都保持了元素的完整性,这表明主要的合金效应是钯原子的稀释,而不是由 d 带移动和填充等全局电子因素造成的。同样,当 Pd 与 Au 合金时,CO、O2、乙烯和乙醛的吸附特性(粘附概率和吸附量)主要受两个原子表面排列变化的影响。金主要起吸附阻滞剂的作用,但程度不同,取决于吸附分子的性质及其对特定集合尺寸的需求。最后,我们考虑了纳米颗粒钯,并概述了合金化对二氧化碳和 H2 高压合成甲醇的影响。钯本身的选择性不强,相反,它主要产生 CO 和甲烷。然而,通过在 ZnO、Ga2O3 和 In2O3 等氧化物上进行支撑,以及在氢气中进行还原,钯会形成合金,从而对甲醇产生高选择性。这也是由于表面的钯集合体被稀释,从而产生了甲烷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


