Triad photosensitizers based on iodo-aza-Bodipy and naphthalenediimides (NDI) with broadband absorption: Study of resonance energy transfer and electron transfer
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Two organic photosensitizers based on resonance energy transfer (A-1 and A-2) are prepared. Naphthalenediimides was used as intramolecular energy donor and iodo-aza-Bodipy was used as intramolecular energy acceptor/spin converter. A-1 (ε = 42,800 at 618 nm and ε = 59,600 at 674 nm) and A-2 (ε = 33,300 at 514 nm and ε = 68,300 at 674 nm) give the two strong absorption band and both of them show broadband absorption in visible region. The photophysical properties of the compounds were studied with steady state and time-resolved spectroscopy in detail. With steady state and time-resolved spectroscopy, we found that photoexcitation into the energy donor was followed by singlet energy transfer, then via the intersystem crossing (ISC) of the energy acceptor. By nanosecond time-resolved transient absorption spectroscopy and DFT calculation, triplet excited state is localized on the iodo-aza-Bodipy moiety. Quantum yield of singlet oxygen of A-1 and A-2 is 39.7 % and 40.9 %, respectively. Based on fluorescence lifetime quenching, the efficiency of intramolecular energy transfer is estimated to be 15.9 % and 27.7 % for A-1 and A-2. And PET is responsible for the relatively low efficiency, which is identified by the calculation of kcs/kFRET and the Gibbs free energy change (△G0cs). A-1 and A-2 were used for singlet oxygen (1O2) mediated photooxidation of 1,5-dihydroxylnaphthalene and the photosensitizing ability are more efficient than the triplet photosensitizers with the mono-chromophore photosensitizer.
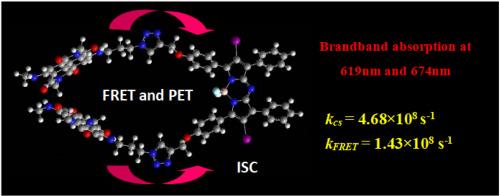
基于碘-氮-波地平和具有宽带吸收的萘二亚胺(NDI)的三元光敏剂:共振能量转移和电子转移研究
制备了两种基于共振能量转移的有机光敏剂(A-1 和 A-2)。萘二甲酰亚胺用作分子内能量供体,碘-氮-联苯胺用作分子内能量受体/自旋转换器。A-1 (ε = 42,800 波长,波长 618 纳米;ε = 59,600 波长,波长 674 纳米)和 A-2(ε = 33,300 波长,波长 514 纳米;ε = 68,300 波长,波长 674 纳米)给出了两个强吸收带,并且都在可见光区域显示出宽带吸收。利用稳态光谱和时间分辨光谱详细研究了这些化合物的光物理特性。通过稳态光谱和时间分辨光谱,我们发现光激发进入能量供体后会发生单线能量转移,然后通过能量受体的系统间交叉(ISC)进行能量转移。通过纳秒时间分辨瞬态吸收光谱和 DFT 计算,三重激发态被定位在碘-氮-联二吡分子上。A-1 和 A-2 的单线态氧量子产率分别为 39.7% 和 40.9%。根据荧光寿命淬灭,估计 A-1 和 A-2 分子内能量转移的效率分别为 15.9% 和 27.7%。通过计算 kcs/kFRET 和吉布斯自由能变化(△G0cs),可以确定 PET 是造成相对较低效率的原因。A-1和A-2用于单线态氧(1O2)介导的1,5-二羟基萘的光氧化,其光敏能力比单色素光敏剂的三重光敏剂更有效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


