Surface science studies on electron-induced reactions of NH3 and their perspectives for enhancing nanofabrication processes
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Ammonia (NH3) dissociates efficiently when it interacts with an electron beam. This applies not only to single electron-NH3 collisions in the gas phase but also to electron irradiation of NH3 adsorbed on surfaces. The dissociation products include atomic hydrogen which can act as a reducing agent or NH2 radicals that can bind to suitable surfaces or to adsorbed molecules. This chemistry can be exploited in nanofabrication processes that use electron beams for deposition, etching, or modification of materials. This review describes the current state of insight regarding electron-induced reactions of NH3 adsorbed on surfaces and outlines approaches to the use of these reactions for enhancing electron beam induced nanofabrication processes. First, an overview of surface science studies on electron-induced reactions of NH3 adsorbed on single crystal surfaces is given. This is followed by a summary of studies on the use of NH3 for improving the purity of deposits prepared by electron beam induced deposition (EBID) and on the prospects of NH3 to suppress unwanted thermal surface chemistry during EBID. Finally, we discuss electron-induced reactions of NH3 that are fundamental to the modification of carbonaceous nanomaterials as well as potential application scenarios such as the functionalization of self-assembled monolayers and humidity sensing.
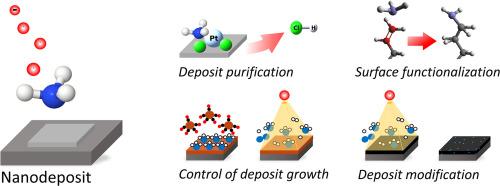
有关 NH3 电子诱导反应的表面科学研究及其在改进纳米制造工艺方面的前景
当氨气(NH3)与电子束发生作用时,它能有效地解离。这不仅适用于气相中的单电子-NH3 碰撞,也适用于电子辐照吸附在表面上的 NH3。解离产物包括可作为还原剂的原子氢或可与适当表面或吸附分子结合的 NH2 自由基。在使用电子束对材料进行沉积、蚀刻或改性的纳米制造工艺中,可以利用这种化学反应。本综述介绍了有关表面吸附的 NH3 电子诱导反应的最新研究成果,并概述了利用这些反应增强电子束诱导的纳米制造工艺的方法。首先,概述了关于单晶表面吸附 NH3 的电子诱导反应的表面科学研究。随后,概述了利用 NH3 提高电子束诱导沉积 (EBID) 制备的沉积物纯度的研究,以及 NH3 在 EBID 过程中抑制不必要的热表面化学反应的前景。最后,我们讨论了 NH3 的电子诱导反应,这些反应对于碳质纳米材料的改性以及自组装单层的功能化和湿度传感等潜在应用场景至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


