Chemical profiling of fungal metabolites via the OSMAC approach: Novel identification of Brianthein W from an endophytic fungus, Hypomontagnella monticulosa Zg15SU
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
Abstract
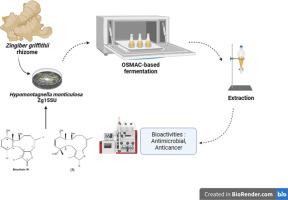
In the previous report, we reported that Hypomontagnella monticulosa originating from the rhizome of Zingiber griffithii was known to produce a marine-derived natural product. An OSMAC-based approach was designed by modifying the nutritional composition of the growth medium to investigate any possible new metabolites produced by the strain. The culture filtrate and biomass were conditioned through the use of three basal media, namely, Czapek-dox, potato dextrose, and Wickerham broth medium. GC–MS and multivariate analysis was performed to distinguish the chemicals and determine their composition in the tested extracts. Antimicrobial activity was tested against selected human pathogenic microbes using the disk-diffusion method. The MeOH extract of both culture filtrate and biomass from different fermentation media revealed that the majority of identified compounds (n = 40) were found in Wickerham medium (n = 23), which is later termed as MeOH-Wi. The chemical composition of MeOH-Wi was fatty acids (21.74 %), followed by terpenoids (17.38 %), cyclosiloxane (13.04 %), aldehydes (13.04 %), alkenes (8.7 %), hydrocarbons (8.7 %), esters (8.7 %), alkaloids (4.35 %), and an unclassified compound, the 9,9-Dimethyl-3,7-diazabicyclo[3.3.1]nonane. The numerous chemical compounds in MeOH-Wi corresponded with its antimicrobial activities against Micrococcus luteus NBRC 13,867 (±11 mm), Candida maltosa NBRC 1977 (±9 mm), and Escherichia coli JM 109 (±5 mm) which were higher than the other tested extracts. Further purification using HPLC RP-C18 using NP-SiO2/n-Hex–EtOAc (10:2) to yield compound (1). Compound (1) was determined as an analog of briarian W—a diterpene mostly found in marine sponge, Briareum spp.—as a pure compound in the MeOH extract of H. monticulosa based on the 1D and 2D NMR analysis. The anticancer activity (IC50) of compound (1) was 0.077, 0.080, and 0.102 µg/mL against the growth of HCT-116, NBT-T2, and Panc-1, respectively. As far as we are aware, this is the first report on finding a briarian diterpene that originates from an endophytic fungus, especially by H. monticulosa.
通过 OSMAC 方法对真菌代谢物进行化学分析:从一种内生真菌--Hypomontagnella monticulosa Zg15SU--中鉴定出新的 Brianthein W
在之前的报告中,我们曾报道过源于格里菲斯茨菰(Zingiber griffithii)根茎的单胞茨菰(Hypomontagnella monticulosa)可产生一种源自海洋的天然产物。我们设计了一种基于 OSMAC 的方法,通过改变生长培养基的营养成分来研究该菌株可能产生的新代谢物。通过使用三种基础培养基(即 Czapek-dox、马铃薯葡萄糖和 Wickerham 肉汤培养基)调节培养滤液和生物量。通过气相色谱-质谱(GC-MS)和多元分析来区分测试提取物中的化学物质并确定其成分。采用磁盘扩散法测试了对某些人类病原微生物的抗菌活性。从不同发酵培养基中提取的培养滤液和生物质的 MeOH 提取物显示,大多数已鉴定化合物(n = 40)存在于 Wickerham 培养基(n = 23)中,这种培养基后来被称为 MeOH-Wi。MeOH-Wi 的化学成分是脂肪酸(21.74 %),其次是萜类(17.38 %)、环硅氧烷(13.04 %)、醛类(13.04 %)、烯类(8.7 %)、烃类(8.7 %)、酯类(8.7 %)、生物碱(4.35 %)和一种未分类的化合物--9,9-二甲基-3,7-二氮杂双环[3.3.1]壬烷。MeOH-Wi 中的多种化合物与其对黄体微球菌 NBRC 13,867 (±11 mm)、麦芽念珠菌 NBRC 1977 (±9 mm)和大肠杆菌 JM 109 (±5 mm)的抗菌活性相对应,其抗菌活性高于其他测试提取物。使用 NP-SiO2/n-Hex-EtOAc (10:2) 进行 HPLC RP-C18 进一步纯化,得到化合物 (1)。根据 1D 和 2D NMR 分析,确定化合物 (1) 是 H. monticulosa 的 MeOH 提取物中的一种纯化合物,它是一种二萜类似物,主要存在于海洋海绵 Briareum spp.中。化合物(1)对 HCT-116、NBT-T2 和 Panc-1 的抗癌活性(IC50)分别为 0.077、0.080 和 0.102 µg/mL。据我们所知,这是首次发现源于内生真菌,尤其是单胞菌的褐藻二萜的报告。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


