Complementary: Green catalysis over red soil for pollutant removal
IF 5.3
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Organic dyes from industrial wastewater pollute water tables. Here proposed solution for environmental control relies on pristine red soil for adsorption and oxidative degradation of methylene blue. Soil analysis comprised XRD, spectroscopic (FTIR and Raman) and microscopic (SEM/EDS) techniques, while spectrophotometry was applied for dye quantification. The dominant soil mineral is kaolinite while Fe homogeneous distribution is witnessed in the α- and γ-FeO(OH) form. Dye adsorption was monitored for both soil and kaolinite, with rising removal under basic conditions due to methylene blue transformation. Theoretical modelling enabled insight into dye orientation over goethite crystallographic planes and estimation of binding energy. Soil/Fenton reagent achieved a substantial 93 % dye removal. An optimal oxidant concentration in the Fenton system was 10 mM. We confirmed the excellent performance of pristine red soil samples as naturally occurring adsorbents and catalysts in Fenton oxidation of environmental pollutants.
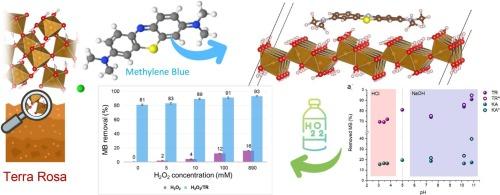
互补:红壤上的绿色催化去除污染物
工业废水中的有机染料会污染地下水。这里提出的环境控制解决方案依赖于原始红土对亚甲基蓝的吸附和氧化降解。土壤分析包括 XRD、光谱(傅立叶变换红外光谱和拉曼光谱)和显微镜(扫描电镜/电子显微镜)技术,而分光光度法则用于染料定量。主要的土壤矿物是高岭石,而铁则以α-和γ-FeO(OH)的形式均匀分布。对土壤和高岭石的染料吸附进行了监测,在碱性条件下,由于亚甲基蓝的转化,染料的去除率上升。理论建模有助于深入了解染料在网纹石晶面上的取向,并估算出结合能。土壤/芬顿试剂对染料的去除率高达 93%。Fenton 系统中的最佳氧化剂浓度为 10 mM。我们证实了原始红土样品作为天然吸附剂和催化剂在芬顿氧化环境污染物中的卓越性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


