Structural analysis of the stable form of fibroblast growth factor 2 – FGF2-STAB
IF 5.1
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
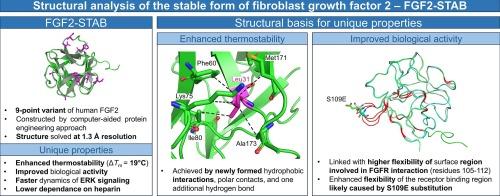
Fibroblast growth factor 2 (FGF2) is a signaling protein that plays a significant role in tissue development and repair. FGF2 binds to fibroblast growth factor receptors (FGFRs) alongside its co-factor heparin, which protects FGF2 from degradation. The binding between FGF2 and FGFRs induces intracellular signaling pathways such as RAS-MAPK, PI3K-AKT, and STAT. FGF2 has strong potential for application in cell culturing, wound healing, and cosmetics but the potential is severely limited by its low protein stability. The thermostable variant FGF2-STAB was constructed by computer-assisted protein engineering to overcome the natural limitation of FGF2. Previously reported characterization of FGF2-STAB revealed an enhanced ability to induce MAP/ERK signaling while having a lower dependence on heparin when compared with FGF2-wt. Here we report the crystal structure of FGF2-STAB solved at 1.3 Å resolution. Protein stabilization is achieved by newly formed hydrophobic interactions, polar contacts, and one additional hydrogen bond. The overall structure of FGF2-STAB is similar to FGF2-wt and does not reveal information on the experimentally observed lower dependence on heparin. A noticeable difference in flexibility in the receptor binding region can explain the differences in signaling between FGF2-STAB and its wild-type counterpart. Our structural analysis provided molecular insights into the stabilization and unique biological properties of FGF2-STAB.
成纤维细胞生长因子 2(FGF2-STAB)稳定形式的结构分析
成纤维细胞生长因子 2(FGF2)是一种信号蛋白,在组织发育和修复中发挥着重要作用。FGF2 与成纤维细胞生长因子受体(FGFRs)结合,其辅助因子肝素可保护 FGF2 免受降解。FGF2 与 FGFRs 之间的结合可诱导细胞内信号通路,如 RAS-MAPK、PI3K-AKT 和 STAT。FGF2 在细胞培养、伤口愈合和美容方面有很大的应用潜力,但由于其蛋白质稳定性较低,应用潜力受到严重限制。为了克服 FGF2 的天然限制,我们通过计算机辅助蛋白质工程构建了恒温变体 FGF2-STAB。与 FGF2-wt 相比,FGF2-STAB 诱导 MAP/ERK 信号转导的能力更强,同时对肝素的依赖性更低。蛋白质的稳定是通过新形成的疏水相互作用、极性接触和一个额外的氢键实现的。FGF2-STAB 的整体结构与 FGF2-wt 相似,并没有揭示实验观察到的肝素依赖性较低的信息。受体结合区灵活性的明显差异可以解释 FGF2-STAB 与野生型受体之间信号传导的差异。我们的结构分析提供了有关 FGF2-STAB 稳定性和独特生物特性的分子见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Structural Biology: X
Biochemistry, Genetics and Molecular Biology-Structural Biology
CiteScore
6.50
自引率
0.00%
发文量
20
审稿时长
62 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


