Charge polarity inversion and zincophilicity improvement for chitosan separator towards durable aqueous zinc-ion batteries
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Aqueous zinc-ion batteries encounter enormous challenges such as Zn dendrites and parasitic reactions. Separator modification is a highly effective strategy to address these issues. With the advantages of low cost, nontoxicity, biodegradability, good film-forming ability, superior hydrophilicity, and rich functional groups, chitosan is an ideal matrix for constructing separators. However, the presence of positive charges within chitosan in weakly acidic electrolytes is unfavorable for dendrite inhibition. Herein, Schiff base reaction is introduced to modify chitosan matrix, transforming its charge polarity from positive to negative. Additionally, NbN with excellent zincophilicity is coated onto chitosan matrix, forming a Janus separator with low thickness of 19 μm and considerably improved mechanical properties. The resultant separator can promote the transport of Zn2+ ions while triggering a repulsive shielding effect against anions, therefore dramatically enhancing Zn2+ ion transfer number from 0.28 to 0.49. This separator can also facilitate desolvation process, improve exchange current density, restrict two-dimensional Zn2+ ion diffusion, and enhance electrochemical kinetics, contributing to significantly inhibited dendrite growth, by-product formation, and hydrogen evolution. Consequently, stable and reversible Zn stripping/plating process is enabled for over 2500 h at 2 mA cm−2 and 2 mAh cm−2. And great rate capability and excellent cyclability can be achieved for full batteries even under harsh conditions. This work provides new insights into separator design for Zn-based batteries.
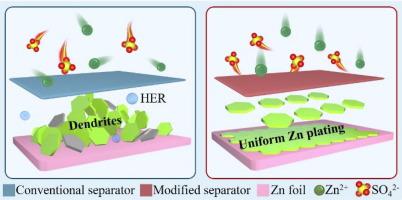
改善壳聚糖隔膜的电荷极性反转和亲锌性,实现耐用的水性锌离子电池
锌离子水电池面临着锌枝晶和寄生反应等巨大挑战。隔膜改性是解决这些问题的一种高效策略。壳聚糖具有成本低、无毒、可生物降解、成膜性好、亲水性强、官能团丰富等优点,是构建隔膜的理想基质。然而,壳聚糖在弱酸性电解质中存在正电荷,不利于抑制树枝状突起。在此,我们引入希夫碱反应来改变壳聚糖基质,将其电荷极性从正极转变为负极。此外,还在壳聚糖基体上涂覆了亲锌性极佳的氮化铌,从而形成了一种厚度仅为 19 μm 的简纳斯分离器,并大大提高了其机械性能。由此产生的分离器可促进 Zn2+ 离子的传输,同时引发对阴离子的排斥屏蔽效应,从而将 Zn2+ 离子转移数从 0.28 大幅提高到 0.49。这种分离器还能促进脱溶过程,提高交换电流密度,限制二维 Zn2+ 离子扩散,并增强电化学动力学,从而显著抑制枝晶生长、副产物形成和氢演化。因此,在 2 mA cm-2 和 2 mAh cm-2 的条件下,可实现超过 2500 小时的稳定、可逆的锌剥离/电镀过程。即使在苛刻的条件下,也能为全电池提供强大的速率能力和出色的循环能力。这项工作为锌基电池的隔膜设计提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


