Nickel-copper alloying arrays realizing efficient co-electrosynthesis of adipic acid and hydrogen
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Constructing electrocatalytic overall reaction technology to couple the electrosynthesis of adipic acid with energy-saving hydrogen production is of significant for sustainable energy systems. However, the development of highly-active bifunctional electrocatalysts remains a challenge. Herein, 3D hierarchical nickel-copper alloying arrays with dendritic morphology are manufactured by a simple electrodeposition process, standing for the excellent bifunctional electrocatalyst towards the co-production of adipic acid and H2 from cyclohexanone and water. The membrane-free flow electrolyzer of Cu0.81Ni0.19/NF shows the superior electrooxidation performance of ketone-alcohol (KA) oil with high faradaic efficiencies of over 90% for adipic acid and H2, robust stability over 200 h as well as a high yield of 0.6 mmol h−1 for adipic acid at 100 mA cm−2. In-situ spectroscopy indicates the Cu0.81Ni0.19 alloy contributes to forming more active NiOOH species to involve in the conversion of cyclohexanone to adipic acid, while the proposed reaction pathway undergoes the 2-hydroxycyclohexanone and 2,7-oxepanedione intermediates. Moreover, the theoretical calculations confirm that the optimal electronic interaction, boosted reaction kinetics as well as improved adsorption free energy of reaction intermediates, synergistically endows Cu0.81Ni0.19 alloy with superior bifunctional performance.
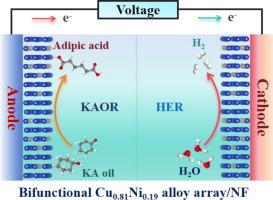
实现己二酸与氢高效共电解的镍铜合金阵列
构建电催化整体反应技术,将己二酸的电合成与节能制氢结合起来,对于可持续能源系统具有重要意义。然而,开发高活性双功能电催化剂仍是一项挑战。本文通过简单的电沉积工艺制备了具有树枝状形态的三维分层镍铜合金阵列,为从环己酮和水联合生产己二酸和 H2 提供了优异的双功能电催化剂。Cu0.81Ni0.19/NF 无膜流动电解槽显示出卓越的酮醇油(KA)电氧化性能,己二酸和 H2 的远红外效率高达 90% 以上,稳定性超过 200 小时,在 100 mA cm-2 的条件下,己二酸的产率高达 0.6 mmol h-1。原位光谱显示,Cu0.81Ni0.19 合金有助于形成更活跃的 NiOOH 物种,参与环己酮到己二酸的转化,而所提出的反应途径则经历了 2-hydroxycyclohexanone 和 2,7-oxepanedione 中间体。此外,理论计算证实,最佳的电子相互作用、反应动力学的提高以及反应中间产物吸附自由能的改善,协同赋予了 Cu0.81Ni0.19 合金卓越的双功能性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


