Dual-phase interface engineering via parallel modulation strategy for highly reversible Zn metal batteries
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
The reversibility and stability of aqueous Zn metal batteries (AZMBs) are largely limited by Zn dendrites and interfacial parasitic reactions. Herein, we propose a parallel modulation strategy to boost the reversibility of the Zn anode by introducing N,N,N’,N’-tetramethylchloroformamidinium hexafluorophosphate (TCFH) as an additive in the electrolyte. TCFH is composed of PF6− and TN+ with opposite charges. PF6− can spontaneously induce the in-situ generation of ZnF2 solid electrolyte interface (SEI) on the anode, which can improve the transport kinetics of Zn2+ at the interface, thus promoting the rapid and uniform deposition of Zn as well as inhibiting the growth of dendrites. In addition, TN+ is enriched at the anode surface during Zn deposition through the anchoring effect, which brings a reconfiguration of the ion/molecule distribution. The anchored-TN+ reduces the concentrations of H2O and SO42−, sufficiently restraining the parasitic reaction. Thanks to the dual-phase interface engineering constructed of PF6− and TN+ in parallel, the symmetric cell with the proposed electrolyte survives long cycling stability over 750 h at 20 mA cm−2, 10 mAh cm−2. This study offers a distinct viewpoint to the multidimensional optimization of Zn anodes for high-performance AZMBs.
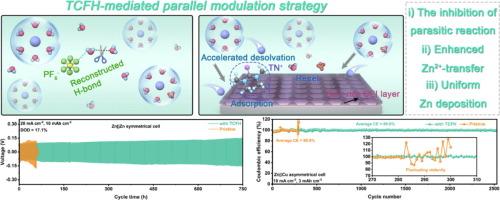
通过并行调制策略实现双相界面工程,打造高度可逆的金属锌电池
水性锌金属电池(AZMB)的可逆性和稳定性在很大程度上受到锌枝晶和界面寄生反应的限制。在此,我们提出了一种并行调制策略,通过在电解液中引入 N,N,N',N'-四甲基氯甲脒六氟磷酸盐(TCFH)作为添加剂来提高锌阳极的可逆性。TCFH 由带相反电荷的 PF6- 和 TN+ 组成。PF6- 能在阳极上自发地诱导原位生成 ZnF2 固体电解质界面(SEI),从而改善 Zn2+ 在界面上的传输动力学,促进 Zn 的快速均匀沉积,并抑制枝晶的生长。此外,在 Zn 沉积过程中,TN+ 通过锚定效应富集在阳极表面,从而带来离子/分子分布的重新配置。锚定的 TN+ 降低了 H2O 和 SO42- 的浓度,充分抑制了寄生反应。得益于 PF6- 和 TN+并行构建的双相界面工程,使用所提议的电解质的对称电池在 20 mA cm-2 和 10 mAh cm-2 条件下可长期稳定循环 750 小时。这项研究为高性能 AZMB 的锌阳极的多维优化提供了一个独特的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


