Adsorption of CO2 on mechanochemically synthesized silicon oxycarbide composites
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The present study explores the mechanochemical synthesis of porous silicon oxycarbide composites using commercial activated carbon and hydrated silica as precursors without additional functionalization, aiming to be used as effective adsorbents for carbon dioxide removal from gas streams. By adjusting the hydrated silica/activated carbon mass ratio, a set of materials were prepared with varying surface areas (256–662 m2/g), pore volumes (0.28–0.45 cm3/g), and surface functional groups concentrations (0.824–0.996 mmol/g). The optimal silicon oxycarbide composite (hydrated silica/activated carbon mass ratio of 1.5) demonstrated the CO2 adsorption capacity of 3.41 mmol/g at 25 °C and 1 bar, which can be attributed to the composite’s well-developed porosity and high surface functionality. In addition, the adsorbent exhibited high CO2/N2 selectivity of 15.2 along with stable cycling performance. These findings indicate that mechanochemically synthesized silicon oxycarbide composites have a considerable potential in the field of CO2 capture.
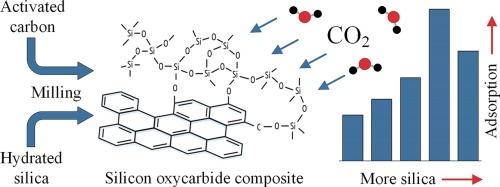
二氧化碳在机械化学合成的碳氧化合物硅复合材料上的吸附作用
本研究探讨了以商用活性炭和水合二氧化硅为前驱体,在不进行额外功能化的情况下,机械化学合成多孔碳氧硅复合材料的方法,旨在将其用作去除气流中二氧化碳的有效吸附剂。通过调整水合二氧化硅/活性炭的质量比,制备出了一组具有不同表面积(256-662 m2/g)、孔体积(0.28-0.45 cm3/g)和表面官能团浓度(0.824-0.996 mmol/g)的材料。最佳碳氧硅复合材料(水合二氧化硅/活性炭质量比为 1.5)在 25 °C 和 1 bar 条件下的二氧化碳吸附容量为 3.41 mmol/g,这归功于复合材料发达的孔隙率和高表面官能团。此外,该吸附剂还具有 15.2 的 CO2/N2 高选择性和稳定的循环性能。这些研究结果表明,机械化学合成的氧碳化硅复合材料在二氧化碳捕集领域具有相当大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


