Casein network formation at oil–water interfaces is reduced by β-casein and increased by Ca2+
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Mixtures of bovine caseins can serve as a benchmark for understanding the functionality of microbial-based recombinant caseins at oil–water interfaces. In this work we show that, in the presence of Ca, the individual casein fractions form viscoelastic networks at the oil–water interface with comparable stiffness. In the absence of Ca, - and -casein interfacial network formation was strongly inhibited over the full deformation regime. For -casein, the network stiffness was increased in the absence of Ca at small deformations (15%), but at large deformations (50%) it was completely disrupted, to a similar stiffness as - and -casein. The interfacial structure formed by -casein was largely unaffected by Ca due to limited phosphorylation. We hypothesize that the differences between calcium-sensitive caseins lie in the conformation they assume at the interface. Both - and -casein adsorb in a train-tail conformation with a tail extending into the aqueous bulk phase, whereas -casein adsorbs in a loop-train conformation, with a loop that extends less into the bulk phase. The tail-train configuration is hypothesized to increase the inter-molecular Ca bridging thereby increasing the interfacial stiffness of - and -casein.
Blending the casein fractions revealed a strong negative effect of -casein on the interfacial modulus, which was more pronounced at a higher concentration. The presence of Ca remained important for interfacial network formation of a casein blend. Without Ca, the interfacial network was less stiff, more viscous, and behaved like a 2d polymer solution.
With this work we showed that casein interfacial network formation at oil–water interfaces is mediated by Ca bridging. Blending the different casein fractions decreased the interfacial viscoelastic properties through the presence of -casein. These results indicate that future work on recombinant caseins should focus on single genetic variants, since a blend of variants will likely decrease interfacial functionality.
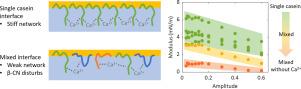
β-酪蛋白会减少油水界面上酪蛋白网络的形成,而 Ca2+ 则会增加这种网络的形成。
牛酪蛋白混合物可作为了解基于微生物的重组酪蛋白在油水界面功能的基准。在这项工作中,我们发现在 Ca2+ 存在的情况下,单个酪蛋白组分在油水界面上形成的粘弹性网络具有可比的刚度。在没有 Ca2+ 的情况下,αs2- 和 β-酪蛋白界面网络的形成在整个变形过程中受到强烈抑制。对于αs1-酪蛋白,在没有 Ca2+ 的情况下,小变形(15%)时网络刚度增加,但大变形(50%)时网络刚度被完全破坏,刚度与αs2-和β-酪蛋白相似。由于磷酸化作用有限,κ-酪蛋白形成的界面结构基本上不受 Ca2+ 的影响。我们推测,钙敏感酪蛋白之间的差异在于它们在界面上的构象。αs2-和β-酪蛋白都以火车尾构象吸附,尾部延伸到水体相中,而αs1-酪蛋白则以环状火车构象吸附,环状部分较少延伸到水体相中。将酪蛋白组分混合后发现,β-酪蛋白对界面模量有很强的负面影响,浓度越高,这种影响越明显。Ca2+ 的存在对酪蛋白混合物界面网络的形成仍然很重要。这项研究表明,油水界面上酪蛋白界面网络的形成是由 Ca2+ 桥接介导的。通过混合不同的酪蛋白组分,β-酪蛋白的存在降低了界面粘弹性。这些结果表明,未来的重组酪蛋白研究工作应侧重于单一基因变体,因为混合变体可能会降低界面功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


