Rheological study of pullulan-pectin mixtures to prepare gel beads using the drip method and evaluation as gallic acid release systems
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
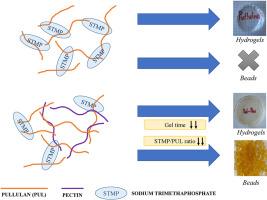
Pullulan (PUL) is a polysaccharide that can form gels using sodium trimetaphosphate (STMP). This gelation occurs at basic pH and has very slow kinetics, so that gel beads cannot be formed by the dripping method since PUL diffuses towards the medium before it can gel. To accelerate the gelling process and be able to obtain pullulan beads, hybrid gels were formed, mixing PUL with pectin (PEC). Then, STMP was added to gel the PUL, and Ca2+ was separately added to gel the PEC for comparative purposes. In both cases, the minimum concentration of gelling agent to obtain a gel was reduced by 90%. The gelation of these STMP-hybrid gels was practically instantaneous, thus allowing the production of beads by dripping the polysaccharide solution in an aqueous STMP solution. The PUL-PEC gels were characterized by FT-IR, which confirmed the junction of the PUL-PEC molecules by hydrogen bonds in the gel, as well as de-esterification of methoxyl groups and ionization of acid groups. The addition of PEC did not affect the swelling degree of the PUL gel, probably since it was governed by STMP-PUL junctions, but it increased the water holding capacity, which was related to the presence of more -OH groups to retain water by hydrogen bonds. PUL-PEC beads loaded with gallic acid were successfully prepared by the dripping method to evaluate their suitability as delivery systems for active ingredients. The release profiles show that they were systems comparable to beads formed by other biopolymers and therefore capable of adequately releasing active ingredients.
利用滴注法制备凝胶珠的拉普兰-pectin 混合物流变学研究以及作为没食子酸释放系统的评估
普鲁兰(PUL)是一种多糖,可以利用三偏磷酸钠(STMP)形成凝胶。这种凝胶化发生在碱性 pH 值条件下,其动力学过程非常缓慢,因此无法通过滴注法形成凝胶珠,因为 PUL 在凝胶化之前会向介质扩散。为了加速凝胶化过程并获得拉普兰珠,将 PUL 与果胶(PEC)混合形成混合凝胶。然后,添加 STMP 使 PUL 凝胶化,并单独添加 Ca2+ 使 PEC 凝胶化,以进行比较。在这两种情况下,获得凝胶的最低胶凝剂浓度都降低了 90%。这些 STMP 杂交凝胶的凝胶化几乎是瞬间完成的,因此可以通过在 STMP 水溶液中滴入多糖溶液来生产珠子。傅立叶变换红外光谱(FT-IR)对 PUL-PEC 凝胶进行了表征,证实了 PUL-PEC 分子通过凝胶中的氢键连接,以及甲氧基的去酯化和酸基的离子化。PEC 的添加并不影响 PUL 凝胶的溶胀度,这可能是因为它受 STMP-PUL 连接的支配,但它增加了持水能力,这与存在更多的 -OH 基团通过氢键保持水分有关。通过滴注法成功制备了负载没食子酸的 PUL-PEC 珠子,以评估其作为活性成分递送系统的适用性。释放曲线显示,它们是与其他生物聚合物形成的珠子相当的系统,因此能够充分释放活性成分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


